Investigating the Impact of Gadolinium-Based Contrast Agents on the Corrected QT Interval
- PMID: 32923263
- PMCID: PMC7485915
- DOI: 10.7759/cureus.9668
Investigating the Impact of Gadolinium-Based Contrast Agents on the Corrected QT Interval
Abstract
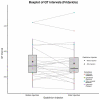
Introduction The manufacturing labels for all currently marketed gadolinium-based MRI contrast agents describe adverse cardiac events reported during post-market use. The goal of this study was to determine prolongation of the rate-corrected QT interval occurs in the immediate setting after gadolinium-based MRI contrast agent injection. Methods This study enrolled adults scheduled to have a gadolinium-based MRI contrast agent injection as part of a diagnostic MRI. A single-lead electrocardiogram was recorded using the AliveCor Kardia® ECG (Mountain View, CA) device before and after injection. The rate-corrected QT interval was subsequently measured by two independent investigators. The QT interval was corrected for rate using the two most common formulas, originally cited by Bazett and Fridericia. These rate-corrected QT intervals from before and after gadolinium-based MRI contrast agent injection were compared using the Wilcoxon signed-rank test paired analysis. Results A total of 24 consenting adults had electrocardiogram that were free of motion artifact. The mean age of the final patient cohort was 59.4 years. There was an equal split of 12 men and 12 women. The mean pre-injection, rate-corrected QT interval, corrected using Bazett's formula, was 395 msec. The mean post-injection, rate-corrected QT interval, corrected using Bazett's formula, was 396 msec. The corrections using Fridericia's formula were 384 and 381 msec, respectively. There was no statistically significant change in Bazett-corrected QT interval (QTc-B) when pre-injection and post-injection values were directly compared. Discussion The results of the present investigation support the conclusion that gadolinium-based MRI contrast agents do not commonly affect rate-corrected QT interval in routine clinical use. While the frequency of rate-corrected QT interval prolongation might be overstated, the severity of adverse events is definitively not. A role for concomitant rate-corrected QT interval-prolonging drugs or unidentified rare factors such as genetic predisposition cannot be ruled out. The limitations of this study include its relatively small size and the implementation of a single-lead electrocardiogram to measure rate-corrected QT interval. Conclusion The present investigation revealed that significant rate-corrected QT interval prolongation, while previously reported in as many as 55% of patients after gadolinium-based MRI contrast agent injection, is not a common occurrence in the routine clinical setting.
Keywords: arrhythmia; drug-induced qtc prolongation; electrocardiogram; gadolinium-based contrast agent; pharmacogenetics.
Copyright © 2020, Gress et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
The best QT correction formula in a non-hospitalized population: the Fasa PERSIAN cohort study.BMC Cardiovasc Disord. 2022 Feb 16;22(1):52. doi: 10.1186/s12872-022-02502-2. BMC Cardiovasc Disord. 2022. PMID: 35172723 Free PMC article.
-
Bazett and Fridericia QT correction formulas interfere with measurement of drug-induced changes in QT interval.Heart Rhythm. 2006 Sep;3(9):1003-7. doi: 10.1016/j.hrthm.2006.05.023. Epub 2006 Jun 15. Heart Rhythm. 2006. PMID: 16945790
-
Literature review and pilot studies of the effect of QT correction formulas on reported beta2-agonist-induced QTc prolongation.Clin Ther. 2006 Apr;28(4):582-90. doi: 10.1016/j.clinthera.2006.04.010. Clin Ther. 2006. PMID: 16750469 Clinical Trial.
-
Rate-corrected QT interval: techniques and limitations.Am J Cardiol. 1993 Aug 26;72(6):17B-22B. doi: 10.1016/0002-9149(93)90035-b. Am J Cardiol. 1993. PMID: 8256750 Review.
-
Ciprofloxacin does not Prolong the QTc Interval: A Clinical Study in ICU Patients and Review of the Literature.J Pharm Pharm Sci. 2017;20(1):360-364. doi: 10.18433/J3ZD15. J Pharm Pharm Sci. 2017. PMID: 29145929 Review.
References
-
- Chelates of gadolinium and dysprosium as contrast agents for MR imaging. Rocklage SM, Watson AD. J Magn Reson Imaging. 1993;3:167–178. - PubMed
-
- Gadolinium retention and toxicity: an update. Ramalho M, Ramalho J, Burke LM, Semelka RC. Adv Chronic Kidney Dis. 2017;24:138–146. - PubMed
-
- Bracco Diagnostics Inc. Multihance (gadobenate dimeglumine) [package insert]. U.S. Food and Drug Administration website. [Mar;2020 ];https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021357s014,021... 2018 505:1–22.
-
- The stretch-activated ion channel blocker gadolinium also blocks L-type calcium channels in isolated ventricular myocytes of the guinea-pig. Lacampagne A, Gannier F, Argibay J, Garnier D, Le Guennec JY. Biochim Biophys Acta. 1994;1191:205–208. - PubMed
-
- Spurious hypocalcemia after gadodiamide-enhanced magnetic resonance imaging:a case report and review of the literature. Moore CD, Newman RC, Caridi JG. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1578545/ Rev Urol. 2006;8:165–168. - PMC - PubMed
LinkOut - more resources
Full Text Sources
