Engineering bacterial outer membrane vesicles as transdermal nanoplatforms for photo-TRAIL-programmed therapy against melanoma
- PMID: 32923586
- PMCID: PMC7455490
- DOI: 10.1126/sciadv.aba2735
Engineering bacterial outer membrane vesicles as transdermal nanoplatforms for photo-TRAIL-programmed therapy against melanoma
Abstract
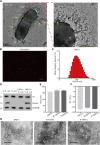
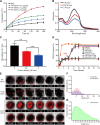
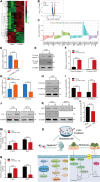
Melanoma is an aggressive cancer with rapid progression, relapse, and metastasis. Systemic therapies for melanoma exhibit limited anticancer potential and high toxicity. Here, we developed the outer membrane vesicles derived from transgenic Escherichia coli, modified with αvβ3 integrin peptide targeting ligand and indocyanine green (named as I-P-OMVs), to induce the transdermal photo-TRAIL-programmed treatment in skin melanoma.-OMVs, which are outer membrane vesicles derived from transgenic Escherichia coli, modified with αvβ3 integrin targeting ligand and indocyanine green (named as I-P-OMVs), to induce the transdermal photo-TRAIL-programmed treatment in skin melanoma. I-P-OMVs exhibited excellent stratum corneum penetration and specificity to melanoma. Upon near-infrared irritation, I-P-OMVs not only induced photothermal-photodynamic responses against primary melanoma spheroids but also activated TRAIL-induced apoptosis in disseminated tumor cells, resulting in a complete eradication of melanoma. I-P-OMVs are the first nanoplatforms to induce transdermal photo-TRAIL-programmed therapy in melanoma with enhanced antitumor performance and high safety, having great potential in cancer therapy.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- Nam J., Son S., Park K. S., Zou W., Shea L. D., Moon J. J., Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 4, 398–414 (2019).
-
- Hersey P., Zhang X. D., How melanoma cells evade trail-induced apoptosis. Nat. Rev. Cancer 1, 142–150 (2001). - PubMed
-
- Gu Z., Biswas A., Zhao M., Tang Y., Tailoring nanocarriers for intracellular protein delivery. Chem. Soc. Rev. 40, 3638–3655 (2011). - PubMed
-
- Chen L., Valentine J. L., Huang C.-J., Endicott C. E., Moeller T. D., Rasmussen J. A., Fletcher J. R., Boll J. M., Rosenthal J. A., Dobruchowska J., Wang Z., Heiss C., Azadi P., Putnam D., Trent M. S., Jones B. D., DeLisa M. P., Outer membrane vesicles displaying engineered glycotopes elicit protective antibodies. Proc. Natl. Acad. Sci. U.S.A. 113, E3609–E3618 (2016). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

