Ingestible transiently anchoring electronics for microstimulation and conductive signaling
- PMID: 32923616
- PMCID: PMC7455191
- DOI: 10.1126/sciadv.aaz0127
Ingestible transiently anchoring electronics for microstimulation and conductive signaling
Abstract
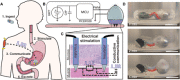
Ingestible electronic devices enable noninvasive evaluation and diagnosis of pathologies in the gastrointestinal (GI) tract but generally cannot therapeutically interact with the tissue wall. Here, we report the development of an orally administered electrical stimulation device characterized in ex vivo human tissue and in in vivo swine models, which transiently anchored itself to the stomach by autonomously inserting electrically conductive, hooked probes. The probes provided stimulation to the tissue via timed electrical pulses that could be used as a treatment for gastric motility disorders. To demonstrate interaction with stomach muscle tissue, we used the electrical stimulation to induce acute muscular contractions. Pulses conductively signaled the probes' successful anchoring and detachment events to a parenterally placed device. The ability to anchor into and electrically interact with targeted GI tissues controlled by the enteric nervous system introduces opportunities to treat a multitude of associated pathologies.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution License 4.0 (CC BY).
Figures




References
-
- Abell T., McCallum R., Hocking M., Koch K., Abrahamsson H., LeBlanc I., Lindberg G., Konturek J., Nowak T., Quigley E. M. M., Tougas G., Starkebaum W., Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology 125, 421–428 (2003). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

