Fate mapping via CCR2-CreER mice reveals monocyte-to-microglia transition in development and neonatal stroke
- PMID: 32923636
- PMCID: PMC7449686
- DOI: 10.1126/sciadv.abb2119
Fate mapping via CCR2-CreER mice reveals monocyte-to-microglia transition in development and neonatal stroke
Abstract
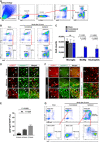
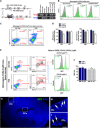
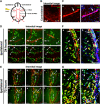
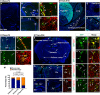
Whether monocytes contribute to the brain microglial pool in development or after brain injury remains contentious. To address this issue, we generated CCR2-CreER mice to track monocyte derivatives in a tamoxifen-inducible manner. This method labeled Ly6Chi and Ly6Clo monocytes after tamoxifen dosing and detected a surge of perivascular macrophages before blood-brain barrier breakdown in adult stroke. When dosed by tamoxifen at embryonic day 17 (E17), this method captured fetal hematopoietic cells at E18, subdural Ki67+ ameboid cells at postnatal day 2 (P2), and perivascular microglia, leptomeningeal macrophages, and Iba1+Tmem119+P2RY12+ parenchymal microglia in selective brain regions at P24. Furthermore, this fate mapping strategy revealed an acute influx of monocytes after neonatal stroke, which gradually transformed into a ramified morphology and expressed microglial marker genes (Sall1, Tmem119, and P2RY12) for at least 62 days after injury. These results suggest an underappreciated level of monocyte-to-microglia transition in development and after neonatal stroke.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Sierra A., Paolicelli R. C., Kettenmann H., Cien Años de Microglía: Milestones in a century of microglial research. Trends Neurosci. 42, 778–792 (2019). - PubMed
-
- Hoeffel G., Chen J., Lavin Y., Low D., Almeida F. F., See P., Beaudin A. E., Lum J., Low I., Forsberg E. C., Poidinger M., Zolezzi F., Larbi A., Ng L. G., Chan J. K. Y., Greter M., Becher B., Samokhvalov I. M., Merad M., Ginhoux F., C-Myb+ erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42, 665–678 (2015). - PMC - PubMed
-
- Prinz M., Jung S., Priller J., Microglia biology: One century of evolving concepts. Cell 179, 292–311 (2019). - PubMed
-
- Ginhoux F., Guilliams M., Tissue-resident macrophage ontogeny and homeostasis. Immunity 44, 439–449 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

