Global and local envelope protein dynamics of hepatitis C virus determine broad antibody sensitivity
- PMID: 32923643
- PMCID: PMC7449684
- DOI: 10.1126/sciadv.abb5938
Global and local envelope protein dynamics of hepatitis C virus determine broad antibody sensitivity
Abstract
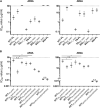
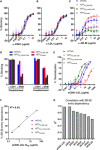
Broad antibody sensitivity differences of hepatitis C virus (HCV) isolates and their ability to persist in the presence of neutralizing antibodies (NAbs) remain poorly understood. Here, we show that polymorphisms within glycoprotein E2, including hypervariable region 1 (HVR1) and antigenic site 412 (AS412), broadly affect NAb sensitivity by shifting global envelope protein conformation dynamics between theoretical "closed," neutralization-resistant and "open," neutralization-sensitive states. The conformational space of AS412 was skewed toward β-hairpin-like conformations in closed states, which also depended on HVR1, assigning function to these enigmatic E2 regions. Scavenger receptor class B, type I entry dependency of HCV was associated with NAb resistance and correlated perfectly with decreased virus propensity to interact with HCV co-receptor CD81, indicating that decreased NAb sensitivity resulted in a more complex entry pathway. This link between global E1/E2 states and functionally distinct AS412 conformations has important implications for targeting AS412 in rational HCV vaccine designs.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Bartenschlager R., Baumert T. F., Bukh J., Houghton M., Lemon S. M., Lindenbach B. D., Lohmann V., Moradpour D., Pietschmann T., Rice C. M., Thimme R., Wakita T., Critical challenges and emerging opportunities in hepatitis C virus research in an era of potent antiviral therapy: Considerations for scientists and funding agencies. Virus Res. 248, 53–62 (2018). - PubMed
-
- Bankwitz D., Steinmann E., Bitzegeio J., Ciesek S., Friesland M., Herrmann E., Zeisel M. B., Baumert T. F., Keck Z.-y., Foung S. K. H., Pécheur E.-I., Pietschmann T., Hepatitis C virus hypervariable region 1 modulates receptor interactions, conceals the CD81 binding site, and protects conserved neutralizing epitopes. J. Virol. 84, 5751–5763 (2010). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

