The Selective Peroxisome Proliferator-Activated Receptor Gamma Modulator CHS-131 Improves Liver Histopathology and Metabolism in a Mouse Model of Obesity and Nonalcoholic Steatohepatitis
- PMID: 32923834
- PMCID: PMC7471426
- DOI: 10.1002/hep4.1558
The Selective Peroxisome Proliferator-Activated Receptor Gamma Modulator CHS-131 Improves Liver Histopathology and Metabolism in a Mouse Model of Obesity and Nonalcoholic Steatohepatitis
Abstract
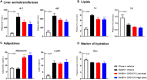
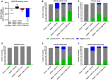
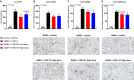
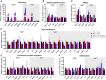
CHS-131 is a selective peroxisome proliferator-activated receptor gamma modulator with antidiabetic effects and less fluid retention and weight gain compared to thiazolidinediones in phase II clinical trials. We investigated the effects of CHS-131 on metabolic parameters and liver histopathology in a diet-induced obese (DIO) and biopsy-confirmed mouse model of nonalcoholic steatohepatitis (NASH). Male C57BL/6JRj mice were fed the amylin liver NASH diet (40% fat with trans-fat, 20% fructose, and 2% cholesterol). After 36 weeks, only animals with biopsy-confirmed steatosis and fibrosis were included and stratified into treatment groups (n = 12-13) to receive for the next 12 weeks (1) low-dose CHS-131 (10 mg/kg), (2) high-dose CHS-131 (30 mg/kg), or (3) vehicle. Metabolic parameters, liver pathology, metabolomics/lipidomics, markers of liver function and liver, and subcutaneous and visceral adipose tissue gene expression profiles were assessed. CHS-131 did not affect body weight, fat mass, lean mass, water mass, or food intake in DIO-NASH mice with fibrosis. CHS-131 improved fasting insulin levels and insulin sensitivity as assessed by the intraperitoneal insulin tolerance test. CHS-131 improved total plasma cholesterol, triglycerides, alanine aminotransferase, and aspartate aminotransferase and increased plasma adiponectin levels. CHS-131 (high dose) improved liver histology and markers of hepatic fibrosis. DIO-NASH mice treated with CHS-131 demonstrated a hepatic shift to diacylglycerols and triacylglycerols with a lower number of carbons, increased expression of genes stimulating fatty acid oxidation and browning, and decreased expression of genes promoting fatty acid synthesis, triglyceride synthesis, and inflammation in adipose tissue. Conclusion: CHS-131 improves liver histology in a DIO and biopsy-confirmed mouse model of NASH by altering the hepatic lipidome, reducing insulin resistance, and improving lipid metabolism and inflammation in adipose tissue.
© 2020 The Authors. Hepatology Communications published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases.
Figures


References
-
- Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2019;69:2672‐2682. - PubMed
-
- Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta‐analysis. J Hepatol 2019;71:793‐801. - PubMed
-
- Buzzetti E, Pinzani M, Tsochatzis EA. The multiple‐hit pathogenesis of non‐alcoholic fatty liver disease (NAFLD). Metabolism 2016;65:1038‐1048. - PubMed
-
- Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz‐Lopez C, et al. Long‐term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med 2016;165:305‐315. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

