Biomarker-Driven Oncology Clinical Trials: Key Design Elements, Types, Features, and Practical Considerations
- PMID: 32923854
- PMCID: PMC7446374
- DOI: 10.1200/PO.19.00086
Biomarker-Driven Oncology Clinical Trials: Key Design Elements, Types, Features, and Practical Considerations
Abstract
In this precision oncology era, where molecular profiling at the individual patient level becomes increasingly accessible and affordable, more and more clinical trials are now driven by biomarkers, with an overarching objective to optimize and personalize disease management. As compared with the conventional clinical development paradigms, where the key is to evaluate treatment effects in histology-defined populations, the choices of biomarker-driven clinical trial designs and analysis plans require additional considerations that are heavily dependent on the nature of biomarkers (eg, prognostic or predictive, integral or integrated) and the credential of biomarkers' performance and clinical utility. Most recently, another major paradigm change in biomarker-driven trials is to conduct multi-agent and/or multihistology master protocols or platform trials. These trials, although they may enjoy substantial infrastructure and logistical advantages, also face unique operational and conduct challenges. Here we provide a concise overview of design options for both the setting of single-biomarker/single-disease and the setting of multiple-biomarker/multiple-disease types. We focus on explaining the trial design and practical considerations and rationale of when to use which designs, as well as how to incorporate various adaptive design components to provide additional flexibility, enhance logistical efficiency, and optimize resource allocation. Lessons learned from real trials are also presented for illustration.
© 2019 by American Society of Clinical Oncology.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center. Chen HuConsulting or Advisory Role: Merck Sharp & DohmeJames J. DignamConsulting or Advisory Role: Merck, Celgene, Northwest Biotherapeutics No other potential conflicts of interest were reported.
Figures
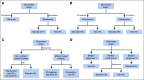
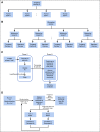
References
-
- Pennello GA. Analytical and clinical evaluation of biomarkers assays: When are biomarkers ready for prime time? Clin Trials. 2013;10:666–676. - PubMed
-
- Dancey JE, Dobbin KK, Groshen S, et al. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin Cancer Res. 2010;16:1745–1755. - PubMed
-
- Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–151. - PubMed
-
- Gehan EA. The determination of the number of patients required in a preliminary and a follow-up trial of a new chemotherapeutic agent. J Chronic Dis. 1961;13:346–353. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

