Precision Oncology and the Universal Health Coverage System in Japan
- PMID: 32923862
- PMCID: PMC7446489
- DOI: 10.1200/PO.19.00291
Precision Oncology and the Universal Health Coverage System in Japan
Abstract
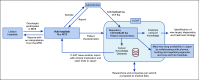
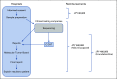
Although precision oncology is transforming clinical management of patients with cancer, many hospitals face challenges to effectively implement precision oncology. In addition, the cost and time exerted for genomic profiling needs to be balanced with expectations of benefit for each patient. This article summarizes the effort to implement precision oncology in Japan. The most promising development is that tests to profile the genomes of select cancers are now fully covered by the national health insurance system. In May 2019, two gene panels were approved with reimbursement: FoundationOne CDx Cancer Genomic Profile and OncoGuide NCC Oncopanel System, the latter of which was developed in Japan. To make better use of scarce resources, the reimbursement is restricted to patients with solid tumors that have progressed on standard chemotherapy, rare tumors, or tumors of unknown primary. To centralize Japanese precision oncology, the government designated approximately 170 hospitals and stratified them to three layers on the basis of their roles. In addition, Japan's National Cancer Center launched a Center for Cancer Genomics and Advanced Therapeutics (C-CAT) that collects genomic information and clinical characteristics of patients who received genomic profiling tests. C-CAT is expected to be the central data repository, to match patients with clinical trials, and to assist translational research. The centralized system under the national health insurance system could be a double-edged sword. Although tight regulation may make it hard to keep up with the rapid development of precision oncology, a federated ecosystem for sharing clinical and genomic data will be a precious asset and allow for shared access to data. Access to unapproved drugs and administrative support from C-CAT will be keys for Japanese precision oncology to meet its full potential.
© 2019 by American Society of Clinical Oncology.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center. Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments). Hiromichi EbiHonoraria: Taiho Pharmaceutical, AstraZeneca Consulting or Advisory Role: Merck Serono, Eli Lilly, Astellas PharmaHideaki BandoHonoraria: Taiho Pharmaceutical, Lilly Japan, Takeda, Chugai Pharma, Sanofi, Yakult Honsha Research Funding: AstraZeneca, Sysmex No other potential conflicts of interest were reported.
Figures

References
-
- Sakamoto H, Rahman M, Nomura S, et al: Japan Health System Review. World Health Organization. Regional Office for South-East Asia. https://apps.who.int/iris/handle/10665/259941.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

