Targeting ERBB2 (HER2) Amplification Identified by Next-Generation Sequencing in Patients With Advanced or Metastatic Solid Tumors Beyond Conventional Indications
- PMID: 32923865
- PMCID: PMC7446516
- DOI: 10.1200/PO.18.00345
Targeting ERBB2 (HER2) Amplification Identified by Next-Generation Sequencing in Patients With Advanced or Metastatic Solid Tumors Beyond Conventional Indications
Abstract
Purpose: Human epidermal growth factor receptor 2 (HER2) is an effective therapeutic target in breast and gastric and gastroesophageal junction cancers. However, less is known about the prevalence of ERBB2 (HER2) amplification and the efficacy of HER2-targeted treatment in other tumors.
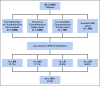
Patients and methods: We assessed HER2 amplification status among 5,002 patients with advanced disease (excluding breast cancer) who underwent next-generation sequencing. We evaluated the clinical benefit of HER2-targeted therapy by measuring the time-dependent overall survival (OS) from the genomic testing results, progression-free survival (PFS), and PFS during HER2-targeted therapy (PFS2) compared with PFS during prior therapy (PFS1).
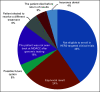
Results: Overall, 122 patients (2.4%) had HER2 amplification, including patients with endometrial (5.3%), bladder (5.2%), biliary or gallbladder (4.9%), salivary (4.7%), and colorectal cancer (3.6%). Forty patients (38%) with nongastric, nongastroesophageal junction, or nonesophageal cancers received at least one line of HER2-targeted therapy. Patients receiving HER2-targeted therapy had a median OS of 18.6 months, compared with 10.9 months for patients who did not receive HER2-targeted therapy (P = .070). On multivariable analysis, HER2-targeted therapy was significantly associated with increased OS (hazard ratio, 0.5; 95% CI, 0.27 to 0.93; P = .029), regardless of sex, age, or number of prior lines of treatment. The PFS2-to-PFS1 ratio was 1.3 or greater in 21 (57%) of 37 patients who received HER2-targeted therapy not in the first line of systemic treatment, and the median PFS2 and PFS1 times were 24 and 13 weeks, respectively (P < .001).
Conclusion: HER2 amplifications using next-generation sequencing can be identified in a variety of tumor types. HER2-targeted therapy may confer clinical benefit in tumor types other than those for which HER2 inhibitors are approved.
© 2019 by American Society of Clinical Oncology.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center. Kavitha BalajiEmployment: Lexicon Stock and Other Ownership Interests: Lexicon Travel, Accommodations, Expenses: LexiconKanwal RaghavHonoraria: Bayer, Eisai Consulting or Advisory Role: Bayer (Inst) Travel, Accommodations, Expenses: TRACON PharmaceuticalsMilind JavleOther Relationship: Rafael Pharmaceuticals, Incyte, Pieris Pharmaceuticals, Merck, Merck Serono, Novartis, Seattle Genetics, BeiGene, QED Therapeutics, BayerMariela Blum-MurphyHonoraria: EMD Serono Research Funding: Bristol-Myers Squibb (Inst), Genentech (Inst)Jaffer AjaniHonoraria: Eli Lilly, Bristol-Myers Squibb, Merck, Aduro Biotech Consulting or Advisory Role: American Cancer Society, BeiGene, Vaccinogen, Insys Therapeutics, Merck, Bristol-Myers Squibb Research Funding: Novartis, Bristol-Myers Squibb, Taiho Pharmaceutical, Genentech, Amgen, Eli Lilly/ImClone, Merck, Delta-Fly Pharma, Gilead Sciences, Takeda, MedImmuneScott KopetzStock and Other Ownership Interests: MolecularMatch, Navire Pharma Consulting or Advisory Role: Roche, Genentech, EMD Serono, Merck, Karyopharm Therapeutics, Amal Therapeutics, Navire Pharma, Symphogen, Holy Stone, Biocartis, Amgen, Novartis, Eli Lilly, Boehringer Ingelheim, Boston Biomedical, AstraZeneca/MedImmune, Bayer Health, Pierre Fabre Research Funding: Amgen (Inst), Sanofi (Inst), Biocartis (Inst), Guardant Health (Inst), Array BioPharma (Inst), Genentech (Inst), EMD Serono (Inst), MedImmune (Inst), Novartis (Inst)Shubham PantHonoraria: 4D Pharma Consulting or Advisory Role: TYME Research Funding: Mirati Therapeutics (Inst), Eli Lilly (Inst), RedHill Biopharma (Inst), Xencor (Inst), Five Prime Therapeutics (Inst), Novartis (Inst), Rgenix (Inst), Sanofi (Inst), ArQule (Inst), Bristol-Myers Squibb (Inst), Onco Response (Inst), GlaxoSmithKline (Inst)Apostolia M. TsimberidouHonoraria: Covance, Genentech Consulting or Advisory Role: Roche Research Funding: EMD Serono (Inst), Baxter (Inst), Foundation Medicine (Inst), Onyx (Inst), Bayer (Inst), Boston Biomedical (Inst), Placon (Inst), Immatics (Inst), Karus Therapeutics (Inst), Stem Cells (Inst), OBI Pharma (Inst) Patents, Royalties, Other Intellectual Property: Parker Institute for Cancer Immunotherapy (Inst)Vivek SubbiahConsulting or Advisory Role: MedImmune Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), NanoCarrier (Inst), Northwest Biotherapeutics (Inst), Genentech (Inst), Berg Pharma (Inst), Bayer (Inst), Incyte (Inst), Fujifilm (Inst), PharmaMar (Inst), D3 Oncology Solutions (Inst), Pfizer (Inst), Amgen (Inst), AbbVie (Inst), Multivir (Inst), Blueprint Medicines (Inst), Loxo (Inst), Vegenics (Inst), Takeda (Inst), Alfasigma (Inst), Agensys (Inst), Idera (Inst), Boston Biomedical (Inst), Inhibrx (Inst), Exelixis (Inst) Travel, Accommodations, Expenses: PharmaMar, BayerDavid S. HongStock and Other Ownership Interests: MolecularMatch, Oncorena, Presagia Honoraria: Adaptimmune, Baxter, Merrimack, Bayer Consulting or Advisory Role: Baxter, Bayer, Guidepoint Global, Janssen, Genentech, Eisai, GLG, Alpha Insights, Axiom Biotechnologies, Adaptimmune, GroupH, Merrimack, Medscape, Numab, Pfizer, Seattle Genetics, Takeda, Trieza Therapeutics Research Funding: Novartis, Genentech, Eisai, AstraZeneca, Pfizer, miRNA Therapeutics, Amgen, Daiichi Sankyo, Merck, Mirati Therapeutics, Eli Lilly, Adaptimmune, AbbVie, Bayer, Bristol-Myers Squibb, Genmab, Ignyta, Infinity Pharmaceuticals, Kite Pharma, Kyowa Hakko Kirin, Loxo, MedImmune, Molecular Templates, Takeda, Seattle Genetics, Amgen, Fate Therapeutics, Mologen, National Cancer Institute Cancer Therapy Evaluation Program Travel, Accommodations, Expenses: Loxo, miRNA Therapeutics, GenmabJordi RodonConsulting or Advisory Role: Novartis, Eli Lilly/ImClone, Servier, Orion Pharma, Peptomyc, Kelun Pharmaceuticals, Merck Sharp & Dohme, Spectrum Pharmaceuticals, Pfizer Research Funding: Novartis, BayerKenna M. ShawResearch Funding: Guardant Health (Inst), Tempus (Inst)Sarina A. Piha-PaulResearch Funding: GlaxoSmithKline (Inst), XuanZhu (Inst), Puma Biotechnology (Inst), Novartis (Inst), Merck Sharp & Dohme (Inst), Curis (Inst), Principa Biopharma (Inst), Helix BioPharma (Inst), Bayer (Inst), AbbVie (Inst), Incyte (Inst), Five Prime Therapeutics (Inst), MedImmune (Inst), Medivation (Inst), BlueLink (Inst), Pfizer (Inst), Tesaro (Inst), Pieris Pharmaceuticals (Inst), Bristol-Myers Squibb (Inst), Samumed (Inst), Aminex (Inst), TransThera (Inst), Genmab (Inst), Seattle Genetics (Inst), Taiho Pharmaceutical (Inst), Cerulean Pharma (Inst), TransThera (Inst), Boehringer Ingelheim (Inst), Chugai Pharma (Inst), Jacobio (Inst)Funda Meric-BernstamHonoraria: Sumitomo Group, Dialectica Consulting or Advisory Role: Genentech, Inflection Biosciences, Pieris Pharmaceuticals, Clearlight Diagnostics, Darwin Health, Samsung Bioepis, Spectrum Pharmaceuticals, Aduro Biotech, Origimed, Xencor, Debiopharm Group, Mersana Research Funding: Novartis, AstraZeneca, Taiho Pharmaceutical, Genentech, Calithera Biosciences, Debiopharm Group, Bayer, Aileron Therapeutics, Puma Biotechnology, CytomX Therapeutics, Jounce Therapeutics, Zymeworks, Curis, Pfizer, eFFECTOR Therapeutics, AbbVie, Boehringer Ingelheim (I), Guardant Health (Inst), Daiichi Sankyo, GlaxoSmithKline No other potential conflicts of interest were reported.
Figures



References
-
- Massard C, Michiels S, Ferté C, et al. High-throughput genomics and clinical outcome in hard-to-treat advanced cancers: Results of the MOSCATO 01 trial. Cancer Discov. 2017;7:586–595. - PubMed
-
- Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19:6102–6114. - PubMed
-
- Hendriks BS, Opresko LK, Wiley HS, et al. Quantitative analysis of HER2-mediated effects on HER2 and epidermal growth factor receptor endocytosis: Distribution of homo- and heterodimers depends on relative HER2 levels. J Biol Chem. 2003;278:23343–23351. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

