Identification of Actionable Genomic Alterations Using Circulating Cell-Free DNA
- PMID: 32923868
- PMCID: PMC7448805
- DOI: 10.1200/PO.19.00017
Identification of Actionable Genomic Alterations Using Circulating Cell-Free DNA
Abstract
Purpose: Cell-free DNA (cfDNA) next-generation sequencing is a noninvasive approach for genomic testing. We report the frequency of identifying alterations and their clinical actionability in patients with advanced/metastatic cancer.
Patients and methods: Prospectively consented patients had cfDNA testing performed. Alterations were assessed for therapeutic implications.
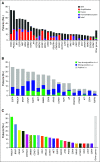
Results: We enrolled 575 patients with 37 tumor types. Of these patients, 438 (76.2%) had at least one alteration detected, and 205 (35.7%) had one or more alterations of high potential for clinical action. In diseases with 10 or more patients enrolled, 50% or more had at least one alteration deemed of high potential for clinical action. Trials were identified in 80% of patients (286 of 357) with any alteration and in 92% of patients (188 of 205) with one or more alterations of high potential for clinical action of whom 57.6% (118 of 205) had 6 or more months of follow-up available. Of these patients, 10% (12 of 118) had received genomically matched therapy through enrollment in clinical trials (n = 8), off-label drug use (n = 3), or standard of care (n = 1). Although 88.6% of all patients had a performance status of 0 or 1 upon enrollment, the primary reason for not acting on alterations was poor performance status at next treatment change (28.1%; 27 of 96).
Conclusion: cfDNA testing represents a readily accessible method for genomic testing and allows for detection of genomic alterations in most patients with advanced disease. Utility may be higher in patients interested in investigational therapeutics with adequate performance status. Additional study is needed to determine whether utility is enhanced by testing earlier in the treatment course.
© 2019 by American Society of Clinical Oncology.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center. Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments). Nora S. SánchezEmployment: Foundation Medicine Stock and Other Ownership Interests: RocheAnn Marie BaileyEmployment: QIAGEN Travel, Accommodations, Expenses: QIAGENKavitha BalajiEmployment: Lexicon Stock and Other Ownership Interests: Lexicon Travel, Accommodations, Expenses: LexiconDong YangEmployment: Molecular HealthMilind JavleConsulting or Advisory Role: QED Therapeutics, Oncosil Medical, Incyte, Mundipharma Other Relationship: Rafael Pharmaceuticals, Incyte, Pieris Pharmaceuticals, Merck, Merck Serono, Novartis, Seattle Genetics, BeiGene, QED Therapeutics, Bayer AGAhmed KasebStock and Other Ownership Interests: Gilead Sciences Honoraria: Merck, Exelixis, Bayer AG, Bristol-Myers Squibb Consulting or Advisory Role: Bayer AG, Bristol-Myers Squibb, Merck, Exelixis Research Funding: Bristol-Myers Squibb, Merck, Bayer AG, Onyx Pharmaceuticals, Genentech Travel, Accommodations, Expenses: Exelixis, Merck, Bayer AG, Onyx Pharmaceuticals, Bristol-Myers SquibbCathy EngHonoraria: Roche, Bayer AG Consulting or Advisory Role: Roche, Genentech, Bayer Schering Pharma, Taiho, Terumo Clinical Supply Travel, Accommodations, Expenses: Genentech, Roche, Bayer AG, Sirtex MedicalVivek SubbiahConsulting or Advisory Role: MedImmune Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), NanoCarrier (Inst), Northwest Biotherapeutics (Inst), Genentech (Inst), Roche (Inst), Berg Pharma (Inst), Bayer AG (Inst), Incyte (Inst), Fujifilm (Inst), PharmaMar (Inst), D3 Oncology Solutions (Inst), Pfizer (Inst), Amgen (Inst), AbbVie (Inst), Multivir (Inst), Blueprint Medicines (Inst), Loxo Oncology (Inst), Vegenics (Inst), Takeda Pharmaceuticals (Inst), Alfasigma (Inst), Agensys (Inst), Idera (Inst), Boston Medical (Inst), Inhibrx (Inst), Exelixis (Inst) Travel, Accommodations, Expenses: PharmaMar, Bayer AGFilip JankuStock and Other Ownership Interests: Trovagene Consulting or Advisory Role: Deciphera, Trovagene, Novartis, Sequenom, Foundation Medicine, Guardant Health, Immunome, Synlogic, Valeant, Dendreon, IFM Therapeutics, Sotio, PureTech Research Funding: Novartis (Inst), BioMed Valley Discoveries (Inst), Roche (Inst), Agios (Inst), Astellas Pharma (Inst), Deciphera (Inst), Plexxikon (Inst), Piqur (Inst), Fujifilm (Inst), Symphogen (Inst), Bristol-Myers Squibb (Inst), Asana Biosciences (Inst), Astex Pharmaceuticals (Inst) Other Relationship: Bio-RadVictoria M. RaymondEmployment: Trovagene, Guardant Health Stock and Other Ownership Interests: Trovagene, Guardant HealthRichard B. LanmanEmployment: Guardant Health, Veracyte Leadership: Guardant Health, Biolase Stock and Other Ownership Interests: Guardant Health, Biolase, Forward Medical Consulting or Advisory Role: Forward Medical Research Funding: Guardant HealthKenna R. Mills ShawResearch Funding: Guardant Health (Inst), Tempus (Inst)Funda Meric-BernstamHonoraria: Sumitomo Group, Dialectica Consulting or Advisory Role: Genentech, Inflection Biosciences, Pieris Pharmaceuticals, Clearlight Diagnostics, Darwin Health, Samsung Bioepis, Spectrum Pharmaceuticals, Aduro Biotech, Origimed, Xencor, Debiopharm Group, Mersana, Seattle Genetics Research Funding: Novartis, AstraZeneca, Taiho Pharmaceutical, Genentech, Calithera Biosciences, Debiopharm Group, Bayer AG, Aileron Therapeutics, Puma Biotechnology, CytomX Therapeutics, Jounce Therapeutics, Zymeworks, Curis, Pfizer, eFFECTOR Therapeutics, AbbVie, Boehringer Ingelheim (I), Guardant Health (Inst), Daiichi Sankyo, GlaxoSmithKline Speakers’ Bureau: Chugai Biopharmaceuticals No other potential conflicts of interest were reported.
Figures



Similar articles
-
Clinical Actionability of Comprehensive Genomic Profiling for Management of Rare or Refractory Cancers.Oncologist. 2016 Nov;21(11):1315-1325. doi: 10.1634/theoncologist.2016-0049. Epub 2016 Aug 26. Oncologist. 2016. PMID: 27566247 Free PMC article.
-
Clinical utility of circulating cell-free DNA in advanced colorectal cancer.PLoS One. 2017 Aug 29;12(8):e0183949. doi: 10.1371/journal.pone.0183949. eCollection 2017. PLoS One. 2017. PMID: 28850629 Free PMC article.
-
High concordance of actionable genomic alterations identified between circulating tumor DNA-based and tissue-based next-generation sequencing testing in advanced non-small cell lung cancer: The Korean Lung Liquid Versus Invasive Biopsy Program.Cancer. 2021 Aug 15;127(16):3019-3028. doi: 10.1002/cncr.33571. Epub 2021 Apr 7. Cancer. 2021. PMID: 33826761
-
Using circulating cell-free DNA to monitor personalized cancer therapy.Crit Rev Clin Lab Sci. 2017 May;54(3):205-218. doi: 10.1080/10408363.2017.1299683. Epub 2017 Apr 10. Crit Rev Clin Lab Sci. 2017. PMID: 28393575 Review.
-
Assessing the Concordance of Genomic Alterations between Circulating-Free DNA and Tumour Tissue in Cancer Patients.Cancers (Basel). 2019 Dec 4;11(12):1938. doi: 10.3390/cancers11121938. Cancers (Basel). 2019. PMID: 31817150 Free PMC article. Review.
Cited by
-
Clinical value of next-generation sequencing in guiding decisions regarding endocrine therapy for advanced HR-positive/HER-2-negative breast cancer.Chin J Cancer Res. 2022 Aug 30;34(4):343-352. doi: 10.21147/j.issn.1000-9604.2022.04.03. Chin J Cancer Res. 2022. PMID: 36199538 Free PMC article.
-
Utility of Germline, Somatic and ctDNA Testing in Adults With Cancer.Cancer Med. 2025 Aug;14(15):e71080. doi: 10.1002/cam4.71080. Cancer Med. 2025. PMID: 40747594 Free PMC article. Review.
-
Circulating tumour DNA in metastatic breast cancer to guide clinical trial enrolment and precision oncology: A cohort study.PLoS Med. 2020 Oct 1;17(10):e1003363. doi: 10.1371/journal.pmed.1003363. eCollection 2020 Oct. PLoS Med. 2020. PMID: 33001984 Free PMC article.
-
The Safety and Suitability of DNA Sequencing of Tissue Biopsies Performed on Patients Referred to a Phase I Unit.Cancers (Basel). 2024 Dec 20;16(24):4252. doi: 10.3390/cancers16244252. Cancers (Basel). 2024. PMID: 39766151 Free PMC article.
-
Leveraging the Fragment Length of Circulating Tumour DNA to Improve Molecular Profiling of Solid Tumour Malignancies with Next-Generation Sequencing: A Pathway to Advanced Non-invasive Diagnostics in Precision Oncology?Mol Diagn Ther. 2021 Jul;25(4):389-408. doi: 10.1007/s40291-021-00534-6. Epub 2021 May 20. Mol Diagn Ther. 2021. PMID: 34018157 Free PMC article. Review.
References
-
- Normanno N, Cervantes A, Ciardiello F, et al. The liquid biopsy in the management of colorectal cancer patients: Current applications and future scenarios. Cancer Treat Rev. 2018;70:1–8. - PubMed
-
- Zill OA, Banks KC, Fairclough SR, et al. The landscape of actionable genomic alterations in cell-free circulating tumor DNA from 21,807 advanced cancer patients. Clin Cancer Res. 2018;24:3528–3538. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

