BRCA Mutations, Homologous DNA Repair Deficiency, Tumor Mutational Burden, and Response to Immune Checkpoint Inhibition in Recurrent Ovarian Cancer
- PMID: 32923884
- PMCID: PMC7446408
- DOI: 10.1200/PO.20.00069
BRCA Mutations, Homologous DNA Repair Deficiency, Tumor Mutational Burden, and Response to Immune Checkpoint Inhibition in Recurrent Ovarian Cancer
Abstract
Purpose: Homologous DNA repair-deficient (HRD) ovarian cancers (OCs), including those with BRCA1/2 mutations, have higher levels of genetic instability, potentially resulting in higher immunogenicity, and have been suggested to respond better to immune checkpoint inhibitors (ICIs) than homologous DNA repair-proficient OCs. However, clinical evidence is lacking. The study aimed to evaluate the associations between BRCA1/2 mutations, HRD, and other genomic parameters and response to ICIs and survival in OC.
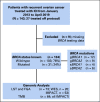
Methods: This is a single-institution retrospective analysis of women with recurrent OC treated with ICIs. BRCA1/2 mutation status and clinicopathologic variables were abstracted from the medical records. Targeted and whole-exome sequencing data available for a subset of patients were used to assess tumor mutational burden (TMB), HRD, and fraction of genome altered (FGA). ICI response was defined as lack of disease progression for ≥ 24 weeks. Associations of BRCA1/2 status and genomic alterations with progression-free survival (PFS) and overall survival (OS) were determined using Cox proportional hazards models.
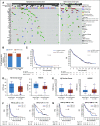
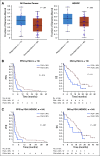
Results: Of the 143 women treated with ICIs, 134 had known BRCA1/2 mutation status. Deleterious germline or somatic BRCA1/2 mutations were present in 31 women (24%). There was no association between presence of BRCA1/2 mutations and response (P = .796) or survival. Genomic analysis in 73 women found no association between TMB (P = .344) or HRD (P = .222) and response, PFS, or OS. There were also no significant differences in somatic genetic alterations between responders and nonresponders. High FGA was associated with an improvement in PFS (P = .014) and OS (P = .01).
Conclusion: TMB, BRCA1/2 mutations, and HRD are not associated with response or survival, cautioning against their use as selection criteria for ICI in recurrent OC. FGA should be investigated further as a biomarker of response to immunotherapy in OC.
© 2020 by American Society of Clinical Oncology.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center. Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments). Alexia IasonosConsulting or Advisory Role: Mylan, Brightpath, IntelligenciaMargaret CallahanEmployment: Bristol Myers Squibb (I), Celgene (I), Kleo Pharmaceuticals (I) Consulting or Advisory Role: AstraZeneca, Moderna Therapeutics, Merck, Immunocore Research Funding: Bristol Myers Squibb (Inst) Other Relationship: Clinical Care Options, Potomac Center for Medical EducationIgnacio Vazquez-GarciaStock and Other Ownership Interests: CRISPR Therapeutics, Editas MedicineAhmet ZehirHonoraria: IlluminaRobert A. BurgerEmployment: Genentech Stock and Other Ownership Interests: Genentech Consulting or Advisory Role: AstraZeneca, Tesaro, Merck, Genentech, Morphotek, Myriad Genetics Research Funding: Incyte (Inst), Astra Zeneca (Inst), and Genzyme (Inst) Travel, Accommodations, Expenses: Tesaro, GenentechDaniel J. Powell JrStock and Other Ownership Interests: Atara Biotherapeutics, InsTIL Bio Consulting or Advisory Role: Neon Therapeutics, Iovance Biotherapeutics, Tmunity Therapeutics, InsTIL Bio, Bellicum Pharmaceuticals Research Funding: Eli Lilly, Tmunity Therapeutics, Incyte, Monojul, AstraZeneca/MedImmune Patents, Royalties, Other Intellectual Property: I hold patents in the field of chimeric antigen receptor (CAR) T-cell therapy in oncology and have received royalties related to their licensing to Novartis; I hold patents in the field of CAR T cell therapy in Oncology and have received royalties related to their licensing to Tmunity Travel, Accommodations, Expenses: Iovance BiotherapeuticsClaire FriedmanConsulting or Advisory Role: AstraZeneca/MedImmune Research Funding: Bristol Myers Squibb (Inst), Arcus Biosciences (Inst), Genentech (Inst), Merck (Inst) Other Relationship: Guidepoint Global Uncompensated Relationships: Genentech, Merck Open Payments Link: https://openpaymentsdata.cms.gov/physician/477023/summaryKaren CadooHonoraria: OncLive Consulting or Advisory Role: GSK Tesaro Research Funding: AstraZeneca (Inst), Syndax (Inst) Travel, Accommodations, Expenses: AstraZenecaRachel GrishamConsulting or Advisory Role: Mateon Therapeutics, Clovis Oncology, Regeneron Research Funding: Context Therapeutics (Inst) Travel, Accommodations, Expenses: EMD Serono Other Relationship: Prime Oncology, MCM Education, OncLiveJason A. KonnerConsulting or Advisory Role: Immunogen, AstraZeneca, Tesaro, AbbVie Research Funding: Genentech Travel, Accommodations, Expenses: AstraZenecaRoisin E. O'CearbhaillConsulting or Advisory Role: Tesaro, GlaxoSmithKline Research Funding: Juno Therapeutics (Inst), Sellas Life Sciences (Inst), Ludwig Institute for Cancer Research (Inst), Stem CentRx (Inst), TapImmune (Inst), TCR2 Therapeutics (Inst), Regeneron (Inst), Genmab (Inst)Carol AghajanianConsulting or Advisory Role: Tesaro, Mersana, Eisai, Roche Research Funding: Genentech (Inst), AbbVie (Inst), Clovis Oncology (Inst), AstraZeneca (Inst), Clovis Oncology (Inst), AstraZeneca (Inst)Jorge S. Reis-FilhoConsulting or Advisory Role: Genentech, Invicro, Ventana Medical Systems, Volition RX, Paige AI, Goldman Sachs, Novartis, Repare TherapeuticsBritta WeigeltConsulting or Advisory Role: Genentech (I), Invicro (I), Ventana Medical Systems (I), Volition RX (I), Page AI (I), Goldman Sachs (I), Repare Therapeutics (I)Dmitriy ZamarinConsulting or Advisory Role: Merck, Synlogic, Western Oncolytics, Tesaro, Agenus, Trieza Therapeutics, ACM Biolabs Research Funding: Genentech (Inst) Patents, Royalties, Other Intellectual Property: I hold a patent regarding the use of recombinant Newcastle disease virus (NDV) for cancer therapy (Inst) No other potential conflicts of interest were reported.
Figures
References
-
- Germano G, Lamba S, Rospo G, et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature. 2017;552:116–120. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

