Metabolic regulation of exercise-induced angiogenesis
- PMID: 32923947
- PMCID: PMC7439921
- DOI: 10.1530/VB-19-0008
Metabolic regulation of exercise-induced angiogenesis
Abstract
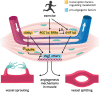
Skeletal muscle relies on an ingenious network of blood vessels, which ensures optimal oxygen and nutrient supply. An increase in muscle vascularization is an early adaptive event to exercise training, but the cellular and molecular mechanisms underlying exercise-induced blood vessel formation are not completely clear. In this review, we provide a concise overview on how exercise-induced alterations in muscle metabolism can evoke metabolic changes in endothelial cells (ECs) that drive muscle angiogenesis. In skeletal muscle, angiogenesis can occur via sprouting and splitting angiogenesis and is dependent on vascular endothelial growth factor (VEGF) signaling. In the resting muscle, VEGF levels are controlled by the estrogen-related receptor γ (ERRγ). Upon exercise, the transcriptional coactivator peroxisome-proliferator-activated receptor-γ coactivator-1α (PGC1α) orchestrates several adaptations to endurance exercise within muscle fibers and simultaneously promotes transcriptional activation of Vegf expression and increased muscle capillary density. While ECs are highly glycolytic and change their metabolism during sprouting angiogenesis in development and disease, a similar role for EC metabolism in exercise-induced angiogenesis in skeletal muscle remains to be elucidated. Nonetheless, recent studies have illustrated the importance of endothelial hydrogen sulfide and sirtuin 1 (SIRT1) activity for exercise-induced angiogenesis, suggesting that EC metabolic reprogramming may be fundamental in this process. We hypothesize that the exercise-induced angiogenic response can also be modulated by metabolic crosstalk between muscle and the endothelium. Defining the underlying molecular mechanisms responsible for skeletal muscle angiogenesis in response to exercise will yield valuable insight into metabolic regulation as well as the determinants of exercise performance.
Keywords: angiogenesis; endothelial metabolism; exercise; metabolism; microvasculature.
© 2019 The authors.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

