GPCR transactivation signalling in vascular smooth muscle cells: role of NADPH oxidases and reactive oxygen species
- PMID: 32923966
- PMCID: PMC7439842
- DOI: 10.1530/VB-18-0004
GPCR transactivation signalling in vascular smooth muscle cells: role of NADPH oxidases and reactive oxygen species
Abstract
The discovery and extension of G-protein-coupled receptor (GPCR) transactivation-dependent signalling has enormously broadened the GPCR signalling paradigm. GPCRs can transactivate protein tyrosine kinase receptors (PTKRs) and serine/threonine kinase receptors (S/TKRs), notably the epidermal growth factor receptor (EGFR) and transforming growth factor-β type 1 receptor (TGFBR1), respectively. Initial comprehensive mechanistic studies suggest that these two transactivation pathways are distinct. Currently, there is a focus on GPCR inhibitors as drug targets, and they have proven to be efficacious in vascular diseases. With the broadening of GPCR transactivation signalling, it is therefore important from a therapeutic perspective to find a common transactivation pathway of EGFR and TGFBR1 that can be targeted to inhibit complex pathologies activated by the combined action of these receptors. Reactive oxygen species (ROS) are highly reactive molecules and they act as second messengers, thus modulating cellular signal transduction pathways. ROS are involved in different mechanisms of GPCR transactivation of EGFR. However, the role of ROS in GPCR transactivation of TGFBR1 has not yet been studied. In this review, we will discuss the involvement of ROS in GPCR transactivation-dependent signalling.
Keywords: G protein; GPCR; TGF-beta; epidermal growth factor; transactivation.
© 2019 The authors.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review. Professor P Little is a Senior Editor of Vascular Biology. Dr D Kamato is an Early Career Researcher on the Editorial Board of Vascular Biology. Professor Little and Dr Kamato were not involved in the review or editorial process for this paper, on which they are listed as authors.
Figures
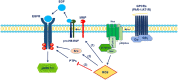
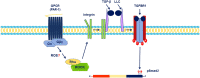
Similar articles
-
Endothelin-1 mediated glycosaminoglycan synthesizing gene expression involves NOX-dependent transactivation of the transforming growth factor-β receptor.Mol Cell Biochem. 2022 Apr;477(4):981-988. doi: 10.1007/s11010-021-04342-8. Epub 2022 Jan 4. Mol Cell Biochem. 2022. PMID: 34982346
-
The expansion of GPCR transactivation-dependent signalling to include serine/threonine kinase receptors represents a new cell signalling frontier.Cell Mol Life Sci. 2015 Feb;72(4):799-808. doi: 10.1007/s00018-014-1775-0. Epub 2014 Nov 12. Cell Mol Life Sci. 2015. PMID: 25384733 Free PMC article. Review.
-
RNA sequencing to determine the contribution of kinase receptor transactivation to G protein coupled receptor signalling in vascular smooth muscle cells.PLoS One. 2017 Jul 18;12(7):e0180842. doi: 10.1371/journal.pone.0180842. eCollection 2017. PLoS One. 2017. PMID: 28719611 Free PMC article.
-
Insights into cellular signalling by G protein coupled receptor transactivation of cell surface protein kinase receptors.J Cell Commun Signal. 2017 Jun;11(2):117-125. doi: 10.1007/s12079-017-0375-9. Epub 2017 Feb 6. J Cell Commun Signal. 2017. PMID: 28168348 Free PMC article. Review.
-
GPCR responses in vascular smooth muscle can occur predominantly through dual transactivation of kinase receptors and not classical Gαq protein signalling pathways.Life Sci. 2013 May 30;92(20-21):951-6. doi: 10.1016/j.lfs.2013.03.017. Epub 2013 Apr 9. Life Sci. 2013. PMID: 23583575 Review.
Cited by
-
Conserved perception of host and non-host signals via the a-pheromone receptor Ste3 in Colletotrichum graminicola.Front Fungal Biol. 2024 Oct 7;5:1454633. doi: 10.3389/ffunb.2024.1454633. eCollection 2024. Front Fungal Biol. 2024. PMID: 39435183 Free PMC article.
-
Vascular smooth muscle cells in intimal hyperplasia, an update.Front Physiol. 2023 Jan 4;13:1081881. doi: 10.3389/fphys.2022.1081881. eCollection 2022. Front Physiol. 2023. PMID: 36685215 Free PMC article. Review.
-
The Role of Toll-like Receptors in Atherothrombotic Cardiovascular Disease.ACS Pharmacol Transl Sci. 2020 Feb 6;3(3):457-471. doi: 10.1021/acsptsci.9b00100. eCollection 2020 Jun 12. ACS Pharmacol Transl Sci. 2020. PMID: 32566912 Free PMC article. Review.
-
Phospholipase C-β3 is dispensable for vascular constriction but indispensable for vascular hyperplasia.Exp Mol Med. 2024 Jul;56(7):1620-1630. doi: 10.1038/s12276-024-01271-6. Epub 2024 Jul 1. Exp Mol Med. 2024. PMID: 38945956 Free PMC article.
-
Lipopolysaccharide acting via toll-like receptor 4 transactivates the TGF-β receptor in vascular smooth muscle cells.Cell Mol Life Sci. 2022 Feb 5;79(2):121. doi: 10.1007/s00018-022-04159-8. Cell Mol Life Sci. 2022. PMID: 35122536 Free PMC article.
References
-
- Kamato D, Rostam MA, Bernard R, Piva TJ, Mantri N, Guidone D, Zheng W, Osman N, Little PJ. The expansion of GPCR transactivation-dependent signalling to include serine/threonine kinase receptors represents a new cell signalling frontier. Cellular and Molecular Life Sciences 2015. 72 . ( 10.1007/s00018-014-1775-0) - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

