Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation
- PMID: 32923970
- PMCID: PMC7439848
- DOI: 10.1530/VB-19-0033
Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation
Abstract
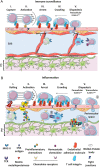
To maintain the homeostatic environment required for proper function of CNS neurons the endothelial cells of CNS microvessels tightly regulate the movement of ions and molecules between the blood and the CNS. The unique properties of these blood vascular endothelial cells are termed blood-brain barrier (BBB) and extend to regulating immune cell trafficking into the immune privileged CNS during health and disease. In general, extravasation of circulating immune cells is a multi-step process regulated by the sequential interaction of adhesion and signalling molecules between the endothelial cells and the immune cells. Accounting for the unique barrier properties of CNS microvessels, immune cell migration across the BBB is distinct and characterized by several adaptations. Here we describe the mechanisms that regulate immune cell trafficking across the BBB during immune surveillance and neuroinflammation, with a focus on the current state-of-the-art in vitro and in vivo imaging observations.
Keywords: blood-brain barrier; immune cell migration; life cell; multiple sclerosis; neuroinflammation.
© 2020 The authors.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

