Tumour vessel remodelling: new opportunities in cancer treatment
- PMID: 32923973
- PMCID: PMC7439841
- DOI: 10.1530/VB-19-0032
Tumour vessel remodelling: new opportunities in cancer treatment
Abstract
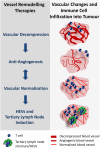
Tumour growth critically depends on a supportive microenvironment, including the tumour vasculature. Tumour blood vessels are structurally abnormal and functionally anergic which limits drug access and immune responses in solid cancers. Thus, tumour vasculature has been considered an attractive therapeutic target for decades. However, with time, anti-angiogenic therapy has evolved from destruction to structural and functional rehabilitation as understanding of tumour vascular biology became more refined. Vessel remodelling or normalisation strategies which alleviate hypoxia are now coming of age having been shown to have profound effects on the tumour microenvironment. This includes improved tumour perfusion, release from immune suppression and lower metastasis rates. Nevertheless, clinical translation has been slow due to challenges such as the transient nature of current normalisation strategies, limited in vivo monitoring and the heterogeneity of primary and/or metastatic tumour environments, calling for more tailored approaches to vascular remodelling. Despite these setbacks, harnessing vascular plasticity provides unique opportunities for anti-cancer combination therapies in particular anti-angiogenic immunotherapy which are yet to reach their full potential.
Keywords: angiogenesis; cancer and tumours; cancer immunotherapy; extracellular matrix; vessel normalisation.
© 2020 The authors.
Conflict of interest statement
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Figures

References
-
- Tannock IF. Population kinetics of carcinoma cells, capillary endothelial cells, and fibroblasts in a transplanted mouse mammary tumor. Cancer Research 1970. 2470–2476. - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. et al Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New England Journal of Medicine 2004. 2335–2342. ( 10.1056/NEJMoa032691) - DOI - PubMed
-
- Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P, Bamias A. et al Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the Aurelia open-label randomized phase III trial. Journal of Clinical Oncology 2014. 1302–1308. ( 10.1200/JCO.2013.51.4489) - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

