The Neurodevelopmental Hypothesis of Huntington's Disease
- PMID: 32925079
- PMCID: PMC7683043
- DOI: 10.3233/JHD-200394
The Neurodevelopmental Hypothesis of Huntington's Disease
Abstract
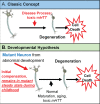
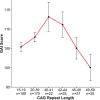
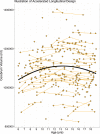
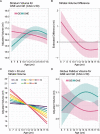
The current dogma of HD pathoetiology posits it is a degenerative disease affecting primarily the striatum, caused by a gain of function (toxicity) of the mutant mHTT that kills neurons. However, a growing body of evidence supports an alternative theory in which loss of function may also influence the pathology.This theory is predicated on the notion that HTT is known to be a vital gene for brain development. mHTT is expressed throughout life and could conceivably have deleterious effects on brain development. The end event in the disease is, of course, neurodegeneration; however the process by which that occurs may be rooted in the pathophysiology of aberrant development.To date, there have been multiple studies evaluating molecular and cellular mechanisms of abnormal development in HD, as well as studies investigating abnormal brain development in HD animal models. However, direct study of how mHTT could affect neurodevelopment in humans has not been approached until recent years. The current review will focus on the most recent findings of a unique study of children at-risk for HD, the Kids-HD study. This study evaluates brain structure and function in children ages 6-18 years old who are at risk for HD (have a parent or grand-parent with HD).
Keywords: Brain development; Huntington’s disease; MRI; children at risk for HD.
Conflict of interest statement
There are no conflicts of interest for any of the authors.
Figures

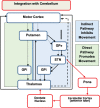

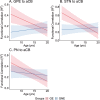
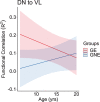
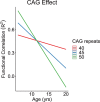

References
-
- Leavitt BR, Tabrizi SJ. Antisense oligonucleotides for neurodegeneration. Science. 2020;367(6485):1428–9. - PubMed
-
- Tabrizi SJ, Leavitt BR, Landwehrmeyer GB, Wild EJ, Saft C, Barker RA, et al. Targeting huntingtin expression in patients with Huntington’s disease. N Engl J Med. 2019;380(24):2307–16. - PubMed
-
- Mehler MF, Gokhan S. Mechanisms underlying neural cell death in neurodegenerative diseases: Alterations of a developmentally-mediated cellular rheostat. Trends Neurosci. 2000;23(12):599–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

