Impact of Low Birth Weight and Prematurity on Neonatal Raltegravir Pharmacokinetics: Impaact P1097
- PMID: 32925360
- PMCID: PMC8043209
- DOI: 10.1097/QAI.0000000000002492
Impact of Low Birth Weight and Prematurity on Neonatal Raltegravir Pharmacokinetics: Impaact P1097
Abstract
Background: HIV treatment of neonates requires identifying appropriate antiretroviral dosing regimens. Our aims were to characterize raltegravir elimination kinetics in low birth weight (LBW) neonates after maternal dosing and to develop a pharmacokinetic model to predict raltegravir plasma concentrations for term and preterm neonates.
Methods: Mothers living with HIV who received raltegravir during pregnancy and their LBW neonates participated in IMPAACT P1097 study. Up to 6 serial plasma samples were collected from each infant over the first 2 postnatal weeks to characterize raltegravir elimination. Safety laboratory evaluations were obtained, and infants were monitored for 6 weeks for signs of raltegravir toxicity. An integrated maternal-neonatal pharmacokinetic model was developed to predict neonatal raltegravir plasma concentrations.
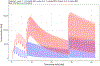
Results: Sixteen mothers and their 18 LBW neonates were enrolled. The median (range) raltegravir elimination half-life was 24.4 (10.1-83) hours (N = 17 neonates). No adverse events related to raltegravir in utero exposure were observed. Pharmacokinetic modeling revealed that raltegravir clearance in full-term LBW neonates was well described by allometric scaling but clearance in preterm LBW neonates was better described using slower clearance maturation kinetics. Simulations suggest receipt of the current dosing recommendations in a 34-week gestation neonate would result in plasma concentrations up to 2.5-fold higher than those observed in full-term LBW infants.
Conclusions: Modeling suggests that prematurity reduces raltegravir clearance and a modified raltegravir dosing regimen will be necessary to avoid elevated plasma raltegravir concentrations.
Figures



References
-
- Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed February 9, 2020.
-
- Capparelli EV, Mirochnick M, Dankner WM, et al. Pharmacokinetics and tolerance of zidovudine in preterm infants. J Pediatr 2003; 142(1): 47–52. - PubMed
-
- Gibango N, Mda S, Ntuli T. Factors associated with delivering premature and/or low birth weight infants among pregnant HIV-positive women on antiretroviral treatment at Dr George Mukhari Hospital, South Africa. Southern African Journal of Infectious Diseases 2018; 33(2): 42–5.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

