Quality of clinical management of children diagnosed with malaria: A cross-sectional assessment in 9 sub-Saharan African countries between 2007-2018
- PMID: 32925906
- PMCID: PMC7489507
- DOI: 10.1371/journal.pmed.1003254
Quality of clinical management of children diagnosed with malaria: A cross-sectional assessment in 9 sub-Saharan African countries between 2007-2018
Abstract
Background: Appropriate clinical management of malaria in children is critical for preventing progression to severe disease and for reducing the continued high burden of malaria mortality. This study aimed to assess the quality of care provided to children under 5 diagnosed with malaria across 9 sub-Saharan African countries.
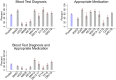
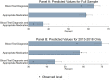
Methods and findings: We used data from the Service Provision Assessment (SPA) survey. SPAs are nationally representative facility surveys capturing quality of sick-child care, facility readiness, and provider and patient characteristics. The data set contained 24,756 direct clinical observations of outpatient sick-child visits across 9 countries, including Uganda (2007), Rwanda (2007), Namibia (2009), Kenya (2010), Malawi (2013), Senegal (2013-2017), Ethiopia (2014), Tanzania (2015), and Democratic Republic of the Congo (2018). We assessed the proportion of children with a malaria diagnosis who received a blood test diagnosis and an appropriate antimalarial. We used multilevel logistic regression to assess facility and provider and patient characteristics associated with these outcomes. Subgroup analyses with the 2013-2018 country surveys only were conducted for all outcomes. Children observed were on average 20.5 months old and were most commonly diagnosed with respiratory infection (47.7%), malaria (29.7%), and/or gastrointestinal infection (19.7%). Among the 7,340 children with a malaria diagnosis, 32.5% (95% CI: 30.3%-34.7%) received both a blood-test-based diagnosis and an appropriate antimalarial. The proportion of children with a blood test diagnosis and an appropriate antimalarial ranged from 3.4% to 57.1% across countries. In the more recent surveys (2013-2018), 40.7% (95% CI: 37.7%-43.6%) of children with a malaria diagnosis received both a blood test diagnosis and appropriate antimalarial. Roughly 20% of children diagnosed with malaria received no antimalarial at all, and nearly 10% received oral artemisinin monotherapy, which is not recommended because of concerns regarding parasite resistance. Receipt of a blood test diagnosis and appropriate antimalarial was positively correlated with being seen at a facility with diagnostic equipment in stock (adjusted OR 3.67; 95% CI: 2.72-4.95) and, in the 2013-2018 subsample, with being seen at a facility with Artemisinin Combination Therapies (ACTs) in stock (adjusted OR 1.60; 95% CI:1.04-2.46). However, even if all children diagnosed with malaria were seen by a trained provider at a facility with diagnostics and medicines in stock, only a predicted 37.2% (95% CI: 34.2%-40.1%) would have received a blood test and appropriate antimalarial (44.4% for the 2013-2018 subsample). Study limitations include the lack of confirmed malaria test results for most survey years, the inability to distinguish between a diagnosis of uncomplicated or severe malaria, the absence of other relevant indicators of quality of care including dosing and examinations, and that only 9 countries were studied.
Conclusions: In this study, we found that a majority of children diagnosed with malaria across the 9 surveyed sub-Saharan African countries did not receive recommended care. Clinical management is positively correlated with the stocking of essential commodities and is somewhat improved in more recent years, but important quality gaps remain in the countries studied. Continued reductions in malaria mortality will require a bigger push toward quality improvements in clinical care.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Malaria Rapid Tests, Febrile Illness Management, and Child Mortality Across Sub-Saharan African Countries.JAMA. 2024 Oct 15;332(15):1270-1281. doi: 10.1001/jama.2024.12589. JAMA. 2024. PMID: 39292453
-
Population coverage of artemisinin-based combination treatment in children younger than 5 years with fever and Plasmodium falciparum infection in Africa, 2003-2015: a modelling study using data from national surveys.Lancet Glob Health. 2017 Apr;5(4):e418-e427. doi: 10.1016/S2214-109X(17)30076-1. Lancet Glob Health. 2017. PMID: 28288746 Free PMC article.
-
Testing times: trends in availability, price, and market share of malaria diagnostics in the public and private healthcare sector across eight sub-Saharan African countries from 2009 to 2015.Malar J. 2017 May 19;16(1):205. doi: 10.1186/s12936-017-1829-5. Malar J. 2017. PMID: 28526075 Free PMC article.
-
Diagnosis and Treatment of the Febrile Child.In: Black RE, Laxminarayan R, Temmerman M, Walker N, editors. Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities, Third Edition (Volume 2). Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2016 Apr 5. Chapter 8. In: Black RE, Laxminarayan R, Temmerman M, Walker N, editors. Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities, Third Edition (Volume 2). Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2016 Apr 5. Chapter 8. PMID: 27227231 Free Books & Documents. Review.
-
Update on malaria.Med Clin (Barc). 2020 Nov 13;155(9):395-402. doi: 10.1016/j.medcli.2020.05.010. Epub 2020 Jun 30. Med Clin (Barc). 2020. PMID: 32620355 Review. English, Spanish.
Cited by
-
Genotyping cognate Plasmodium falciparum in humans and mosquitoes to estimate onward transmission of asymptomatic infections.Nat Commun. 2021 Feb 10;12(1):909. doi: 10.1038/s41467-021-21269-2. Nat Commun. 2021. PMID: 33568678 Free PMC article.
-
Mortality in children and adolescents in Western Democratic Republic of Congo: retrospective analysis of verbal autopsy and demographic data from the Kimpese Health and Demographic Surveillance System.BMJ Paediatr Open. 2025 Mar 22;9(1):e003224. doi: 10.1136/bmjpo-2024-003224. BMJ Paediatr Open. 2025. PMID: 40121016 Free PMC article.
-
How socioeconomic status affected the access to health facilities and malaria diagnosis in children under five years: findings from 19 sub-Saharan African countries.Infect Dis Poverty. 2023 Apr 6;12(1):29. doi: 10.1186/s40249-023-01075-2. Infect Dis Poverty. 2023. PMID: 37024969 Free PMC article.
-
Impact of the Severe Malaria "Champions Program" on the Management of Severe Malaria Cases in 12 Hospitals of the North and Far North Regions of Cameroon.Am J Trop Med Hyg. 2024 Feb 6;110(3_Suppl):76-82. doi: 10.4269/ajtmh.23-0528. Print 2024 Mar 5. Am J Trop Med Hyg. 2024. PMID: 38320307 Free PMC article.
-
Diagnosis and management of malaria in the intensive care unit.J Intensive Med. 2023 Nov 3;4(1):3-15. doi: 10.1016/j.jointm.2023.09.002. eCollection 2024 Jan. J Intensive Med. 2023. PMID: 38263976 Free PMC article. Review.
References
-
- World Health Organization. World malaria report 2019. Geneva, Switzerland: World Health Organization; 2019.
-
- Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin-based combination therapy for treating uncomplicated malaria In: The Cochrane Collaboration, editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2009. http://doi.wiley.com/10.1002/14651858.CD007483.pub2 - DOI - PMC - PubMed

