The causal effect of obesity on prediabetes and insulin resistance reveals the important role of adipose tissue in insulin resistance
- PMID: 32925908
- PMCID: PMC7515203
- DOI: 10.1371/journal.pgen.1009018
The causal effect of obesity on prediabetes and insulin resistance reveals the important role of adipose tissue in insulin resistance
Abstract
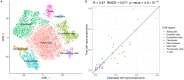
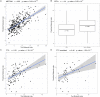
Reverse causality has made it difficult to establish the causal directions between obesity and prediabetes and obesity and insulin resistance. To disentangle whether obesity causally drives prediabetes and insulin resistance already in non-diabetic individuals, we utilized the UK Biobank and METSIM cohort to perform a Mendelian randomization (MR) analyses in the non-diabetic individuals. Our results suggest that both prediabetes and systemic insulin resistance are caused by obesity (p = 1.2×10-3 and p = 3.1×10-24). As obesity reflects the amount of body fat, we next studied how adipose tissue affects insulin resistance. We performed both bulk RNA-sequencing and single nucleus RNA sequencing on frozen human subcutaneous adipose biopsies to assess adipose cell-type heterogeneity and mitochondrial (MT) gene expression in insulin resistance. We discovered that the adipose MT gene expression and body fat percent are both independently associated with insulin resistance (p≤0.05 for each) when adjusting for the decomposed adipose cell-type proportions. Next, we showed that these 3 factors, adipose MT gene expression, body fat percent, and adipose cell types, explain a substantial amount (44.39%) of variance in insulin resistance and can be used to predict it (p≤2.64×10-5 in 3 independent human cohorts). In summary, we demonstrated that obesity is a strong determinant of both prediabetes and insulin resistance, and discovered that individuals' adipose cell-type composition, adipose MT gene expression, and body fat percent predict their insulin resistance, emphasizing the critical role of adipose tissue in systemic insulin resistance.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Chung JO, Cho DH, Chung DJ, Chung MY. Associations among Body Mass Index, Insulin Resistance, and Pancreatic β-Cell Function in Korean Patients with New-Onset Type 2 Diabetes FAU—Chung, Jin Ook FAU Cho, Dong Hyeok FAU—Chung, Dong Jin FAU—Chung, Min Young. Korean J Intern Med. 2012;27(1):66–71. 10.3904/kjim.2012.27.1.66 - DOI - PMC - PubMed
-
- Meah FA, DiMeglio LA, Greenbaum CJ, Blum JS, Sosenko JM, Pugliese A, et al. The relationship between BMI and insulin resistance and progression from single to multiple autoantibody positivity and type 1 diabetes among TrialNet Pathway to Prevention participants. Diabetologia. 2016;59(6):1186–95. 10.1007/s00125-016-3924-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

