Change in Donor Characteristics and Antibodies to SARS-CoV-2 in Donated Blood in the US, June-August 2020
- PMID: 32926078
- PMCID: PMC7490741
- DOI: 10.1001/jama.2020.18598
Change in Donor Characteristics and Antibodies to SARS-CoV-2 in Donated Blood in the US, June-August 2020
Abstract
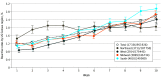
This study describes changes in blood donor demographics and seroreactivity after testing of blood donations for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies began and was publicized in the US in mid-June 2020.
Conflict of interest statement
Figures
References
-
- US Food and Drug Administration Investigational COVID-19 convalescent plasma: guidance for industry. Published April 2020. Updated May 2020. Accessed August 10, 2020. https://www.fda.gov/media/136798/download
-
- US Food and Drug Administration Instructions for use: Vitros Immunodiagnostics Anti-SARS-Cov-2 IgG. Accessed August 10, 2020. https://www.fda.gov/media/137363/download
-
- Tenforde MW, Billig Rose E, Lindsell CJ, et al. ; CDC COVID-19 Response Team . Characteristics of adult outpatients and inpatients with COVID-19: 11 academic medical centers, United States, March-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(26):841-846. doi: 10.15585/mmwr.mm6926e3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

