Association of Prediagnostic Frailty, Change in Frailty Status, and Mortality After Cancer Diagnosis in the Women's Health Initiative
- PMID: 32926116
- PMCID: PMC7490646
- DOI: 10.1001/jamanetworkopen.2020.16747
Association of Prediagnostic Frailty, Change in Frailty Status, and Mortality After Cancer Diagnosis in the Women's Health Initiative
Abstract
Importance: Understanding changes in frailty in relation to cancer diagnosis can inform optimal selection of cancer treatments and survivorship care.
Objective: To investigate associations of prediagnostic frailty and change in frailty status with mortality after a cancer diagnosis.
Design, setting, and participants: This multicenter, prospective cohort study included 7257 community-dwelling, postmenopausal women in the United States who had frailty assessed at the Women's Health Initiative (WHI) enrollment (1993-1998) and the 3-year visit who were subsequently diagnosed as having invasive cancer. The data were analyzed from January 7, 2019, to June, 8, 2020.
Exposure: Frailty scores were defined from validated questionnaire items conceptually aligned with the Fried frailty phenotype, including at least 3 of the following characteristics: self-reported unintentional weight loss, exhaustion, low physical activity, and muscle weakness or impaired walking. Physical function components of the frailty score were updated a median of 10 (range, 1-18) times.
Main outcomes and measures: Using multivariable-adjusted Cox proportional hazards models, this study examined associations of prediagnostic frailty (at the 3-year visit, before cancer diagnosis) and prediagnostic changes in frailty (from enrollment to the 3-year visit) with mortality. Women were followed up beginning from cancer diagnosis for mortality outcomes through March 2018. In linear mixed-effects models with frailty scores as a function of time since cancer diagnosis, this study evaluated whether the time slope, ie, the rate of change in frailty score, increased after cancer diagnosis.
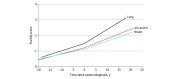
Results: This study included 7257 women in the WHI cohort who completed frailty assessments at enrollment and the 3-year WHI visit before cancer diagnosis and subsequently developed cancer. Cancer cases included 2644 breast cancers (36%), 822 lung cancers (11%), 691 colorectal cancers (10%), 445 endometrial cancers (6%), and 286 ovarian cancers (4%). At the 3-year visit, prior to cancer diagnosis, the mean (SD) age was 63 (7) years, and 1161 of 7257 (16%) of participating women met criteria for frailty; 2129 of 7257 (29%) were prefrail, and 3967 of 7257 (55%) were nonfrail. Over a median follow-up of 5.8 years after cancer diagnosis (range, 1 day to 19.9 years), 3056 women died. After multivariable adjustment, women who were frail (vs nonfrail) before cancer diagnosis had an increased risk of mortality after cancer diagnosis (hazard ratio [HR], 1.40; 95% CI, 1.26-1.55; P for trend <.001). Sustained frailty (21% [1537 of 7257] of women) or worsening frailty (22% [1578 of 7257]) vs being consistently nonfrail (45% [3266 of 7257]) before cancer diagnosis increased the risk of mortality after cancer diagnosis (HR, 1.25; 95% CI, 1.14-1.38 and 1.22; 95% CI, 1.11-1.34, respectively; P for trend <.001). In linear mixed-effects models, the rate of increase in physical frailty over time was statistically significantly higher after cancer diagnosis.
Conclusions and relevance: Sustained and worsening frailty before cancer diagnosis was associated with an increased risk of mortality after cancer diagnosis in postmenopausal women. Furthermore, the rate of decline in physical function accelerated after cancer diagnosis. Frailty assessment could provide valuable information and perhaps prompt interventions to reduce and preempt worsening of physical frailty after cancer diagnosis.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

