Aural toilet (ear cleaning) for chronic suppurative otitis media
- PMID: 32926406
- PMCID: PMC8095014
- DOI: 10.1002/14651858.CD013057.pub2
Aural toilet (ear cleaning) for chronic suppurative otitis media
Update in
-
Aural toilet (ear cleaning) for chronic suppurative otitis media.Cochrane Database Syst Rev. 2025 Jun 9;6(6):CD013057. doi: 10.1002/14651858.CD013057.pub3. Cochrane Database Syst Rev. 2025. PMID: 40484404
Abstract
Background: Chronic suppurative otitis media (CSOM), sometimes referred to as chronic otitis media (COM), is a chronic inflammation and often polymicrobial infection (involving more than one micro-organism) of the middle ear and mastoid cavity, characterised by ear discharge (otorrhoea) through a perforated tympanic membrane. The predominant symptoms of CSOM are ear discharge and hearing loss. Aural toileting is a term describing a number of processes for manually cleaning the ear. Techniques used may include dry mopping (with cotton wool or tissue paper), suction clearance (typically under a microscope) or irrigation (using manual or automated syringing). Dry mopping may be effective in removing mucopurulent discharge. Compared to irrigation or microsuction it is less effective in removing epithelial debris or thick pus. Aural toileting can be used alone or in addition to other treatments for CSOM, such as antibiotics or topical antiseptics.
Objectives: To assess the effects of aural toilet procedures for people with CSOM.
Search methods: The Cochrane ENT Information Specialist searched the Cochrane ENT Register; Central Register of Controlled Trials (CENTRAL via the Cochrane Register of Studies); Ovid MEDLINE; Ovid Embase; CINAHL; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 16 March 2020.
Selection criteria: We included randomised controlled trials (RCTs) with at least a one-week follow-up involving people (adults and children) who had chronic ear discharge of unknown cause or CSOM, where the ear discharge had continued for more than two weeks. We included any aural toileting method as the intervention, at any frequency and for any duration. The comparisons were aural toileting compared with a) placebo or no intervention, and b) any other aural toileting method. We analysed trials in which background treatments were used in both arms (e.g. topical antiseptics or topical antibiotics) separately.
Data collection and analysis: We used the standard Cochrane methodological procedures. We used GRADE to assess the certainty of the evidence for each outcome. Our primary outcomes were: resolution of ear discharge or 'dry ear' (whether otoscopically confirmed or not), measured at between one week and up to two weeks, two weeks to up to four weeks, and after four weeks; health-related quality of life using a validated instrument; and ear pain (otalgia) or discomfort or local irritation. Secondary outcomes were hearing, serious complications, and the adverse events of ear bleeding and dizziness/vertigo/balance problems.
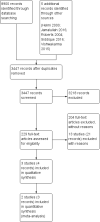
Main results: We included three studies with a total of 431 participants (465 ears), reporting on two comparisons. Two studies included only children with CSOM in the community (351 participants) and the other study (80 participants) included children and adults with chronic ear discharge for at least six weeks. None of the included studies reported the outcomes of health-related quality of life, ear pain or the adverse event of ear bleeding. Daily aural toileting (dry mopping) versus no treatment Two studies (351 children; 370 ears) compared daily dry mopping with no treatment. Neither study presented results for resolution of ear discharge at between one and up to two weeks or between two to four weeks. For resolution of ear discharge after four weeks, one study reported the results per person. We are very uncertain whether there is a difference at 16 weeks (risk ratio (RR) 1.01, 95% confidence interval (CI) 0.60 to 1.72; 1 study; 217 participants) because the certainty of the evidence is very low. No results were reported for the adverse events of dizziness, vertigo or balance problems. Only one study reported serious complications, but it was not clear which group these patients were from, or whether the complications occurred pre- or post-treatment. One study reported hearing, but the results were presented by treatment outcome rather than by treatment group so it is not possible to determine whether there is a difference between the two groups. Daily aural toileting versus single aural toileting on top of topical ciprofloxacin One study (80 participants; 95 ears) compared daily aural toileting (suction) with administration of topical antibiotic (ciprofloxacin) ear drops in a clinic, to a single aural toileting (suction) episode followed by daily self-administered topical antibiotic drops, in participants of all ages. We are unsure whether there is a difference in resolution of ear discharge at between one and up to two weeks (RR 1.09, 95% CI 0.91 to 1.30; 1 study; 80 participants) because the certainty of the evidence is very low. There were no results reported for resolution of ear discharge at between two to four weeks. The results for resolution of ear discharge after four weeks were presented by ear, not person, and could not be adjusted to by person. One patient in the group with single aural toileting and self administration of topical antibiotic ear drops reported the adverse event of dizziness, which the authors attributed to the use of cold topical ciprofloxacin. It is very uncertain whether there is a difference between the groups (RR 0.33, 95% CI 0.01 to 7.95; 1 study; 80 participants, very low-certainty). No results were reported for the other adverse events of vertigo or balance problems, or for serious complications. The authors only reported qualitatively that there was no difference between the two groups in hearing results (very low-certainty).
Authors' conclusions: We are very uncertain whether or not treatment with aural toileting is effective in resolving ear discharge in people with CSOM, due to a lack of data and the poor quality of the available evidence. We also remain uncertain about other outcomes, including adverse events, as these were not well reported. Similarly, we are very uncertain whether daily suction clearance, followed by antibiotic ear drops administered at a clinic, is better than a single episode of suction clearance followed by self-administration of topical antibiotic ear drops.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Mahmood F Bhutta: Professor Mahmood Bhutta has received an honorarium from Novus Therapeutics for advice on an experimental treatment for otitis media (not related to any treatment in this review).
Karen Head: none known.
Lee Yee Chong: none known.
Jessica Daw: none known.
Anne GM Schilder: Professor Anne Schilder was until March 2020 the joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review. Her evidENT team at UCL is supported in part by the National Institute of Health Research (NIHR) University College London Hospitals Biomedical Research Centre. The research is funded by the NIHR and EU Horizon2020. She is the national chair of the NIHR Clinical Research Network ENT Specialty. She is the Surgical Specialty Lead for ENT for the Royal College of Surgeons of England's Clinical Trials Initiative. In her role as director of the NIHR UCLH BRC Deafness and Hearing Problems Theme, she acts as an advisor on clinical trial design and delivery to a range of biotech companies, most currently Novus Therapeutics.
Martin J Burton: Professor Martin Burton is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Christopher G Brennan‐Jones: Dr Brennan‐Jones's research team is primarily funded by the Australian NHMRC and the WA Department of Health. He sits on the national Technical Advisory Group responsible for developing treatment guidelines for otitis media in Australia.
Figures





References
References to studies included in this review
Eason 1986 {published data only}
-
- Eason RJ, Harding E, Nicholson R, Nicholson D, Pada J, Gathercole J. Chronic suppurative otitis media in the Solomon Islands: a prospective, microbiological, audiometric and therapeutic survey. New Zealand Medical Journal 1986;99:812-5. [CENTRAL: CN-00045614] [PMID: ] - PubMed
Kiris 1998 {published data only}
-
- Kiris M, Berktas M, Egeli E, Kutluhan A. The efficacy of topical ciprofloxacin in the treatment of chronic suppurative otitis media. Ear, Nose, & Throat Journal 1998;77(11):904-5, 909. [CENTRAL: CN-00306946] [PMID: ] - PubMed
Smith 1996 {published data only}
-
- Mackenzie IJ, Smith AW, Hatcher J, Machria I. Randomized controlled trial of treatment of chronic suppurative otitis media in Kenyan school children [abstract]. Clinical Otolaryngology 1997;22:81. [CENTRAL: CN-00262473]
-
- Smith AW, Hatcher J, Mackenzie IJ, Thompson S, Bal I, Macharia I, et al. Randomised controlled trial of treatment of chronic suppurative otitis media in Kenyan school children. Lancet 1996;348(9035):1128-33. [CENTRAL: CN-00132594] [EMBASE: 1996327505] [PMID: ] - PubMed
References to studies excluded from this review
Boesorire 2000 unpublished {unpublished data only}
-
- Boesoirie T. A comparative study between ofloxacin ear drop and neomycin-polymixin b-hydrocortisone ear drops on the chronic suppurative otitis media. In: 9th ASEAN ORL Head and Neck Congress 31 March-1 April, 2001. Singapore, 2001.
-
- Boesoirie T. A comparative study between ofloxacin ear drops and neomycin-polymixin b-hydrocortisone ear drops on the chronic suppurative otitis media. Department of ENT Head and Neck Surgery, University of Padjajaran, Bandung, Indonesia Unpublished, 2000.
Browning 1988 {published data only}
-
- Browning GG, Gatehouse S, Calder IT. Medical management of active chronic otitis media: a controlled study. Journal of Laryngology and Otology 1988;102(6):491-5. [CENTRAL: CN-00054939] [PMID: ] - PubMed
Fliss 1990 {published data only}
-
- Fliss DM, Dagan R, Houri Z, Leiberman A. Medical management of chronic suppurative otitis media without cholesteatoma in children. Journal of Pediatrics 1990;116(6):991-6. [CENTRAL: CN-00067997] [PMID: ] - PubMed
-
- Leiberman A, Fliss DM, Dagan R. Medical treatment of chronic suppurative otitis media without cholesteatoma in children-a two-year follow-up. International Journal of Pediatric Otorhinolaryngology 1992;24(1):25-33. [PMID: ] - PubMed
Gendeh 2001 unpublished {unpublished data only}
-
- Gendeh S. A comparative study of ofloxacin otic drops vs framycetin sulfate-dexamethasone-gramicidin otic drops in the medical treatment of otitis externa and chronic suppurative otitis media. In: 9th ASEAN ORL Head and Neck Congress, 31 March-1 April, 2001. Singapore, 2001.
-
- Gendeh S. A comparative study of ofloxacin otic drops vs. framycetin sulfate-dexamethasone-gramicidin otic drops in the medical treatment of otitis externa and chronic suppurative otitis media. Department of ORL, Hospital University Kabangsaan Malaysia, Kuala Lumpur, Malaysia Unpublished, 2000.
Gupta 2015 {published data only}
Helmi 2000 unpublished {unpublished data only}
-
- Helmi A, Ratna D, Zainul A, Sosialisman E, Alfian FH, Bambang H. The efficacy and safety of ofloxacin otic solution for active suppurative otitis media. Faculty of Medicine, University of Indonesia, Jakarta, Indonesia Unpublished, 2000.
-
- Helmi A, et al. The efficacy and safety of ofloxacin otic solution for active suppurative otitis media. In: 9th ASEAN ORL Head and Neck Congress, 31 March-1 April, 2001. Singapore, 2001.
I‐HEAR‐BETA {published data only}
-
- ACTRN12614000234617. Comparing cotrimoxazole and/or povidone-iodine ear wash with standard dry mopping and ciprofloxacin ear drops in Indigenous children with chronic suppurative otitis media (CSOM). Http://www.anzctr.org.au/ACTRN12614000234617.aspx 2014. [CENTRAL: CN-01013236]
-
- Wigger C, Leach AJ, Beissbarth J, Oguoma V, Lennox R, Nelson S, et al. Povidone-iodine ear wash and oral cotrimoxazole for chronic suppurative otitis media in Australian aboriginal children: study protocol for factorial design randomised controlled trial. BMC Pharmacology and Toxicology 2019;20:46. [DOI: 10.1186/s40360-019-0322-x] - DOI - PMC - PubMed
IRCT2016082313136N4 {published data only}
-
- IRCT2016082313136N4. The effect clotrimazole ointment comparison to Ceftizoxime powder and clotrimazole ointment in the treatment of patients with fungal otitis media. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2016082313136N4 2016. [CENTRAL: CN-01810510]
Loock 2012 {published data only}
-
- Loock J. Strategies in the medical treatment of active mucosal chronic otitis media suitable for all levels of healthcare: a randomized controlled trial. Clinical Otolaryngology 2012;37(Suppl 1):165-6. [CENTRAL: CN-01008068] [EMBASE: 71023646]
-
- Loock JW. A randomised controlled trial of active chronic otitis media comparing courses of eardrops versus one-off topical treatments suitable for primary, secondary and tertiary healthcare settings. Clinical Otolaryngology 2012;37(4):261-70. [CENTRAL: CN-00850193] [EMBASE: 365535141] [PMID: ] - PubMed
Minja 2006 {published data only}
-
- Minja BM, Moshi NH, Ingvarsson L, Bastos I, Grenner J. Chronic suppurative otitis media in Tanzanian school children and its effects on hearing. East African Medical Journal 2006;83(6):322-5. [CENTRAL: CN-00568108] [PMID: ] - PubMed
Nwokye 2015 {published data only}
-
- Bakshi SS. Occurrence of otitis media in children and assessment of treatment options. Journal of Laryngology and Otology 2015;129(12):1253. [PMID: ] - PubMed
-
- Nwokoye NN, Egwari LO, Olubi OO. Occurrence of otitis media in children and assessment of treatment options. Journal of Laryngology and Otology 2015;129(8):779-83. [PMID: ] - PubMed
Papastavros 1989 {published data only}
-
- Papastavros T, Giamarellou H, Varlejides S. Preoperative therapeutic considerations in chronic suppurative otitis media. Laryngoscope 1989;99(6 Pt 1):655-9. [CENTRAL: CN-00060222] [PMID: ] - PubMed
Subramaniam 2001 unpublished {unpublished data only}
-
- Subramaniam K, Jalaludin M, Krishnan G. Comparative study of ofloxacin otic drops versus neomycin-polymixin b-hydrocortisone in the medical management of chronic suppurative otitis media. Department of ORL, University Malaya Medical Center, Kuala Lumpur, Malaysia Unpublished, 2000.
-
- Subramaniam K, Jaludin M, Krishnan G. Comparative study of ofloxacin otic drops versus neomycin-polymixin hydrocortisone in the medical management of chronic suppurative otitis media. In: 9th ASEAN ORL Head and Neck Congress, 31 March-1 April, 2001. Singapore, 2001.
Additional references
Addams‐Williams 2010
Baumann 2011
Bhutta 2011
Bhutta 2016
-
- Bhutta MF. Evolution and otitis media: a review, and a model to explain high prevalence in indigenous populations. Human Biology 2016;87(2):92-108. - PubMed
Bhutta 2018
Brennan‐Jones 2018b
Brennan‐Jones 2020
Chong 2018a
Chong 2018b
CONSORT 2010
Dubey 2007
-
- Dubey SP, Larawin V. Complications of chronic suppurative otitis media and their management. Laryngoscope 2007;117(2):264-7. - PubMed
Egger 1997
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. - PubMed
Elemraid 2010
-
- Elemraid MA, Brabin BJ, Fraser WD, Harper G, Faragher B, Atef Z, et al. Characteristics of hearing impairment in Yemeni children with chronic suppurative otitis media: a case-control study. International Journal of Pediatric Otorhinolaryngology 2010;74(3):283-6. - PubMed
Gates 2002
-
- Gates GA, Klein JO, Lim DJ, Mogi G, Ogra PL, Pararella MM, et al. Recent advances in otitis media. 1. Definitions, terminology, and classification of otitis media. Annals of Otology, Rhinology & Laryngology. Supplement 2002;188:8-18. - PubMed
Gray 1988
-
- Gray RF, Nicolaides AR. Vertigo following aural suction: can it be prevented? Clinical Otolaryngology 1988;13:285–8. - PubMed
Handbook 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hannley 2000
-
- Hannley MT, Denneny JC, Holzer SS. Use of ototopical antibiotics in treating 3 common ear diseases. Otolaryngology - Head and Neck Surgery 2000;122(6):934-40. - PubMed
Head 2020a
Head 2020b
Jensen 2013
Kirkham 2017
Mahadevan 2012
Monasta 2012
Morris 2010
-
- Morris P, Leach A, Shah P, Nelson S, Anand A, Allnutt R, et al. Recommendations for Clinical Care Guidelines on the Management of Otitis Media: In Aboriginal and Torres Strait Islander Populations. Canberra: Office for Aboriginal and Torres Strait Islander Health, Australian Government, 2010.
Nadol 2000
Olatoke 2008
-
- Olatoke F, Ologe FE, Nwawolo CC, Saka MJ. The prevalence of hearing loss among school children with chronic suppurative otitis media in Nigeria, and its effect on academic performance. Ear, Nose, & Throat Journal 2008;87(12):E19. - PubMed
Orji 2013
Phillips 2014a
Phillips 2014b
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schilder 2016
Stedman 2011
van der Veen 2006
van Dinther 2015
Verhoeff 2006
-
- Verhoeff M, Veen EL, Rovers MM, Sanders EA, Schilder AG. Chronic suppurative otitis media: a review. International Journal of Pediatric Otorhinolaryngology 2006;70(1):1-12. - PubMed
WHO 2004
-
- World Health Organization. Chronic Suppurative Otitis Media (CSOM): Burden of Illness and Management Options. Geneva, Switzerland: World Health Organization, 2004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

