Randomized clinical trial of negative pressure wound therapy as an adjunctive treatment for small-area thermal burns in children
- PMID: 32926410
- PMCID: PMC7692881
- DOI: 10.1002/bjs.11993
Randomized clinical trial of negative pressure wound therapy as an adjunctive treatment for small-area thermal burns in children
Abstract
Background: The efficacy of negative pressure wound therapy (NPWT) in the acute management of burns remains unclear. The purpose of this trial was to compare standard Acticoat™ and Mepitel™ dressings with combined Acticoat™, Mepitel™ and continuous NPWT to determine the effect of adjunctive NPWT on re-epithelialization in paediatric burns.
Methods: This two-arm, single-centre RCT recruited children with acute thermal burns covering less than 5 per cent of their total body surface area. The primary outcome was time to re-epithelialization. Blinded assessments were performed using photographs captured every 3-5 days until discharge. Secondary measures included pain, itch, grafting, perfusion and scar management referrals.
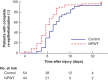
Results: Some 114 patients were randomized. Median time to re-epithelialization was 8 (i.q.r. 7-11) days in the NPWT group and 10 (8-14) days in the control group. In a multivariable model, NPWT decreased the expected time to wound closure by 22 (95 per cent c.i. 7 to 34) per cent (P = 0·005). The risk of referral to scar management was reduced by 60 (18 to 81) per cent (P = 0·013). Four participants in the control group and one in the NPWT group underwent grafting. There were no statistically significant differences between groups in pain, itch or laser Doppler measures of perfusion. Adverse events were rare and minor, although NPWT carried a moderate treatment burden, with ten patients discontinuing early.
Conclusion: Adjunctive NPWT hastened re-epithelialization in small-area burn injuries in children, but had a greater treatment burden than standard dressings alone. Registration number: ACTRN12618000256279 ( http://ANZCTR.org.au).
Antecedentes: La eficacia del tratamiento de las heridas con presión negativa (negative pressure wound therapy, NPWT) en el tratamiento agudo de las quemaduras sigue sin estar claro. El propósito de este ensayo clínico fue comparar los apósitos estándar del tipo Acticoat™ y Mepitel™ con la combinación de Acticoat™, Mepitel™ y NPWT continua para determinar el efecto de la adición de NPWT en la reepitelización de las quemaduras en pediatría. MÉTODOS: Ensayo controlado y aleatorizado, con dos brazos y unicéntrico, que reclutó niños con quemaduras térmicas agudas que afectaban < 5% de la superficie corporal total. El resultado primario fue el tiempo hasta la reepitelización. Se realizaron evaluaciones a ciegas utilizando fotografías tomadas cada 3-5 días hasta el alta hospitalaria. Las medidas secundarias incluían dolor, picor, injerto, perfusión y derivación para el tratamiento de las cicatrices.
Resultados: Se aleatorizaron un total de 114 pacientes. La mediana de tiempo hasta la reepitelización fue 8 días (rango intercuartílico, interquartile range, IQR 7-11) en el grupo NPWT y 10 días (8-14) en el grupo control. En el modelo multivariable, el uso de NPWT disminuyó los días previstos hasta el cierre de la herida en un 22% (i.c. del 95% 7-34%; P = 0,005). El riesgo de ser derivado para el tratamiento de la cicatriz se redujo en un 60% (18-81%; P = 0,013). Cuatro participantes en el grupo control y uno en el grupo NPWT fueron sometidos a injertos. No hubo diferencias estadísticamente significativas en el dolor, picor, o mediciones de la perfusión con Doppler laser. Los eventos adversos fueron raros y menores, aunque NPWT conllevó una carga de tratamiento moderada con 10 pacientes que lo suspendieron precozmente. CONCLUSIÓN: El tratamiento complementario de la herida con presión negativa acelera el tiempo hasta la reepitelización en quemaduras de pequeña extensión en niños, pero implica una mayor carga de tratamiento.
© 2020 The Authors. British Journal of Surgery published by John Wiley & Sons Ltd on behalf of BJS Society Ltd.
Figures
Comment in
-
Correspondence: Comment on 'Randomized clinical trial of negative pressure wound therapy as an adjunctive treatment for small-area thermal burns in children' by Frear et al.Br J Surg. 2021 Mar 12;108(2):e86. doi: 10.1093/bjs/znaa077. Br J Surg. 2021. PMID: 33711130 No abstract available.
-
Author response to: Comment on: "Randomized clinical trial of negative pressure wound therapy as an adjunctive treatment for small-area thermal burns in children" by Frear et al.Br J Surg. 2021 Mar 12;108(2):e87. doi: 10.1093/bjs/znaa078. Br J Surg. 2021. PMID: 33711134 No abstract available.
Similar articles
-
Randomized controlled trial of three burns dressings for partial thickness burns in children.Burns. 2015 Aug;41(5):946-55. doi: 10.1016/j.burns.2014.11.005. Epub 2015 Feb 14. Burns. 2015. PMID: 25687836 Clinical Trial.
-
Study of negative pressure wound therapy as an adjunct treatment for acute burns in children (SONATA in C): protocol for a randomised controlled trial.Trials. 2019 Feb 13;20(1):130. doi: 10.1186/s13063-019-3223-9. Trials. 2019. PMID: 30760332 Free PMC article.
-
Cost-effectiveness of silver dressings for paediatric partial thickness burns: An economic evaluation from a randomized controlled trial.Burns. 2017 Jun;43(4):724-732. doi: 10.1016/j.burns.2016.09.018. Epub 2017 Apr 10. Burns. 2017. PMID: 28408145 Clinical Trial.
-
The efficacy and safety of negative pressure wound therapy in paediatric burns: a systematic review and meta-analysis of randomized controlled trials.BMC Pediatr. 2024 Dec 18;24(1):807. doi: 10.1186/s12887-024-05302-z. BMC Pediatr. 2024. PMID: 39696096 Free PMC article.
-
Negative Pressure Wound Therapy for Burns.Clin Plast Surg. 2017 Jul;44(3):671-677. doi: 10.1016/j.cps.2017.02.023. Epub 2017 Apr 7. Clin Plast Surg. 2017. PMID: 28576256 Review.
Cited by
-
Improving the patient-centred care of children with life-altering skin conditions using feedback from electronic patient-reported outcome measures: protocol for a hybrid effectiveness-implementation study (PEDS-ePROM).BMJ Open. 2021 Apr 9;11(4):e041861. doi: 10.1136/bmjopen-2020-041861. BMJ Open. 2021. PMID: 33837095 Free PMC article.
-
An Overview of Recent Developments in the Management of Burn Injuries.Int J Mol Sci. 2023 Nov 15;24(22):16357. doi: 10.3390/ijms242216357. Int J Mol Sci. 2023. PMID: 38003548 Free PMC article. Review.
-
Technologies used in the treatment of burn victims in intensive care: a scope review.Rev Bras Enferm. 2024 May 13;77(1):e20220738. doi: 10.1590/0034-7167-2022-0738. eCollection 2024. Rev Bras Enferm. 2024. PMID: 38747745 Free PMC article. Review.
-
Implementation of negative pressure for acute pediatric burns (INPREP): A stepped-wedge cluster randomized controlled trial protocol.PLoS One. 2024 Dec 10;19(12):e0315278. doi: 10.1371/journal.pone.0315278. eCollection 2024. PLoS One. 2024. PMID: 39656723 Free PMC article.
-
Stem Cell-Based Tissue Engineering for the Treatment of Burn Wounds: A Systematic Review of Preclinical Studies.Stem Cell Rev Rep. 2022 Aug;18(6):1926-1955. doi: 10.1007/s12015-022-10341-z. Epub 2022 Feb 12. Stem Cell Rev Rep. 2022. PMID: 35150392 Free PMC article.
References
-
- Peck MD. Epidemiology of burns throughout the world. Part I: distribution and risk factors. Burns 2011; 37: 1087–1100. - PubMed
-
- Greenhalgh D. Management of burns. N Engl J Med 2019; 380: 2349–2359. - PubMed
-
- Deitch EA, Wheelahan TM, Rose MP, Clothier J, Cotter J. Hypertrophic burn scars: analysis of variables. J Trauma 1983; 23: 895–898. - PubMed
-
- Lonie S, Baker P, Teixeira RP. Healing time and incidence of hypertrophic scarring in paediatric scalds. Burns 2016; 43: 509–513. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials