Macular OCT Characteristics at 36 Weeks' Postmenstrual Age in Infants Examined for Retinopathy of Prematurity
- PMID: 32927150
- PMCID: PMC7947027
- DOI: 10.1016/j.oret.2020.09.004
Macular OCT Characteristics at 36 Weeks' Postmenstrual Age in Infants Examined for Retinopathy of Prematurity
Abstract
Purpose: To report our ability to capture,-grade reliably, and analyze bedside macular OCT images from preterm infants and relate OCT findings to biological factors and retinopathy of prematurity (ROP) status at a single time window in the Study of Eye Imaging in Preterm Infants (BabySTEPS).
Design: Prospective, observational study.
Participants: Preterm infants eligible for ROP screening with parental consent for research and a 36 ± 1 weeks' postmenstrual age (PMA) visit.
Methods: We imaged both eyes of preterm infants with an investigational noncontact, handheld swept-source (SS) OCT at the time of clinical ROP examinations. Macular OCT features and layer thicknesses for untreated eyes of infants at 36 ± 1 weeks' PMA were compared with demographic data and clinical ROP examination performed by experts. Statistical analyses accounted for the use of both eyes of infants.
Main outcome measures: Macular OCT features and layer thicknesses, gender, race or ethnicity, gestational age, birth weight, ROP stage, and plus disease.
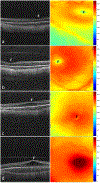
Results: We captured macular OCT from 169 eyes (1 eye excluded because of prior ROP treatment) at 36 ± 1 weeks' PMA. The quality of OCT volumes was excellent in 33 eyes (19%), acceptable in 112 eyes (67%), poor in 24 eyes (14%), and unusable in 0 eyes (0%). Macular edema was present in 60% of eyes and was bilateral in 82% of infants with edema. At the fovea, retinal and inner nuclear layer thickness increased with edema severity: 183 ± 36 μm and 51 ± 27 μm in mild (16% of eyes), 308 ± 57 μm and 163 ± 53 μm in moderate (25%), and 460 ± 76 μm and 280 ± 83 μm in severe edema (12%), respectively. With an increase in ROP stage from 0 to 2, the mean ± standard deviation retinal thickness at the fovea increased from 227± 124 μm to 297 ± 99 μm (P < 0.001). The choroid was thinner, 155 ± 72 μm, with preplus or plus disease versus without, 236 ± 79 μm (P = 0.04), whereas retinal thickness did not vary.
Conclusions: We demonstrated the reliability of methods and the prevalence of OCT findings in preterm infants enrolled in BabySTEPS at a single time point of 36 ± 1 weeks' PMA. Variations in layer thicknesses in infants at this time point may reflect abnormalities resulting from delay in foveal development that may be impacted by macular edema, ROP, or both.
Keywords: BabySTEPS; OCT; Preterm infants; Retinopathy of prematurity; swept-source OCT.
Copyright © 2020 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


References
-
- Patel CK. Optical coherence tomography in the management of acute retinopathy of prematurity. Am J Ophthalmol 2006;141(3):582–4. - PubMed
-
- Vinekar A, Sivakumar M, Shetty R, et al. A novel technique using spectral-domain optical coherence tomography (Spectralis, SD-OCT+HRA) to image supine non-anaesthetized infants: utility demonstrated in aggressive posterior retinopathy of prematurity. Eye (Lond) 2010;24(2):379–82. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

