Genotype-Guided Hydralazine Therapy
- PMID: 32927458
- PMCID: PMC7606720
- DOI: 10.1159/000510433
Genotype-Guided Hydralazine Therapy
Abstract
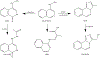
Background: Despite its approval in 1953, hydralazine hydrochloride continues to be used in the management of resistant hypertension, a condition frequently managed by nephrologists and other clinicians. Hydralazine hydrochloride undergoes metabolism by the N-acetyltransferase 2 (NAT2) enzyme. NAT2 is highly polymorphic as approximately 50% of the general population are slow acetylators. In this review, we first evaluate the link between NAT2 genotype and phenotype. We then assess the evidence available for genotype-guided therapy of hydralazine, specifically addressing associations of NAT2 acetylator status with hydralazine pharmacokinetics, antihypertensive efficacy, and toxicity.
Summary: There is a critical need to use hydralazine in some patients with resistant hypertension. Available evidence supports a significant link between genotype and NAT2 enzyme activity as 29 studies were identified with an overall concordance between genotype and phenotype of 92%. The literature also supports an association between acetylator status and hydralazine concentration, as fourteen of fifteen identified studies revealed significant relationships with a consistent direction of effect. Although fewer studies are available to directly link acetylator status with hydralazine antihypertensive efficacy, the evidence from this smaller set of studies is significant in 7 of 9 studies identified. Finally, 5 studies were identified which support the association of acetylator status with hydralazine-induced lupus. Clinicians should maintain vigilance when prescribing maximum doses of hydralazine. Key Messages: NAT2 slow acetylator status predicts increased hydralazine levels, which may lead to increased efficacy and adverse effects. Caution should be exercised in slow acetylators with total daily hydralazine doses of 200 mg or more. Fast acetylators are at risk for inefficacy at lower doses of hydralazine. With appropriate guidance on the usage of NAT2 genotype, clinicians can adopt a personalized approach to hydralazine dosing and prescription, enabling more efficient and safe treatment of resistant hypertension.
Keywords: Hydralazine; N-acetyltransferase 2; Pharmacogenetics; Resistant hypertension.
© 2020 S. Karger AG, Basel.
Conflict of interest statement
Disclosure Statement
The authors have no conflicts of interest to declare.
Figures
References
-
- NCBI. Genetic Testing Registry. [cited 2020 June 8]. Available from: https://www.ncbi.nlm.nih.gov/gtr/all/tests/?term=10[geneid.
-
- Arbor Pharmaceuticals LLC. BiDil [package insert]. [cited 2020 June 8]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/020727s010lbl.pdf. Revised 2019. March 12.
-
- Novartis. Apresoline [package insert]. [cited 2020 June 8]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/1996/008303s068lbl.pdf. Revised 1996. February 28.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

