State of the Art Review on Genetics and Precision Medicine in Arrhythmogenic Cardiomyopathy
- PMID: 32927679
- PMCID: PMC7554944
- DOI: 10.3390/ijms21186615
State of the Art Review on Genetics and Precision Medicine in Arrhythmogenic Cardiomyopathy
Abstract
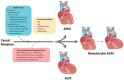
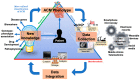
Arrhythmogenic cardiomyopathy (ACM) is an inherited cardiomyopathy characterised by ventricular arrhythmia and an increased risk of sudden cardiac death (SCD). Numerous genetic determinants and phenotypic manifestations have been discovered in ACM, posing a significant clinical challenge. Further to this, wider evaluation of family members has revealed incomplete penetrance and variable expressivity in ACM, suggesting a complex genotype-phenotype relationship. This review details the genetic basis of ACM with specific genotype-phenotype associations, providing the reader with a nuanced perspective of this condition; whilst also proposing a future roadmap to delivering precision medicine-based management in ACM.
Keywords: arrhythmogenic cardiomyopathy; arrhythmogenic right ventricular cardiomyopathy; cardiac arrhythmia; desmosome; genetics; genotype phenotype correlation; sudden cardiac death.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
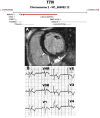
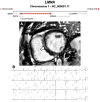
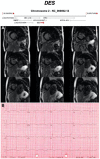
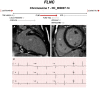
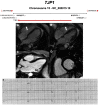
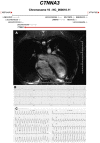
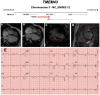
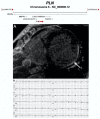
References
-
- Towbin J.A., McKenna W.J., Abrams D.J., Ackerman M.J., Calkins H., Darrieux F.C.C., Daubert J.P., de Chillou C., DePasquale E.C., Desai M.Y., et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16:e301–e372. doi: 10.1016/j.hrthm.2019.05.007. - DOI - PubMed
-
- Marcus F.I., McKenna W.J., Sherrill D., Basso C., Bauce B., Bluemke D.A., Calkins H., Corrado D., Cox M.G., Daubert J.P., et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

