Exhaustion of Protective Heat Shock Response Induces Significant Tumor Damage by Apoptosis after Modulated Electro-Hyperthermia Treatment of Triple Negative Breast Cancer Isografts in Mice
- PMID: 32927720
- PMCID: PMC7565562
- DOI: 10.3390/cancers12092581
Exhaustion of Protective Heat Shock Response Induces Significant Tumor Damage by Apoptosis after Modulated Electro-Hyperthermia Treatment of Triple Negative Breast Cancer Isografts in Mice
Abstract
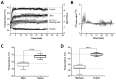
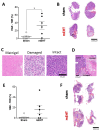
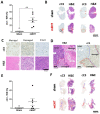
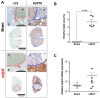
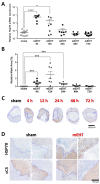
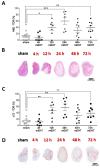
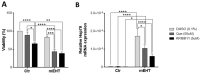
Modulated electro-hyperthermia (mEHT) is a complementary antitumor therapy applying capacitive radiofrequency at 13.56 MHz. Here we tested the efficiency of mEHT treatment in a BALB/c mouse isograft model using the firefly luciferase-transfected triple-negative breast cancer cell line, 4T1. Tumors inoculated orthotopically were treated twice using a novel ergonomic pole electrode and an improved mEHT device (LabEHY 200) at 0.7 ± 0.3 W for 30 min. Tumors were treated one, two, or three times every 48 h. Tumor growth was followed by IVIS, caliper, and ultrasound. Tumor destruction histology and molecular changes using immunohistochemistry and RT-qPCR were also revealed. In vivo, mEHT treatment transitionally elevated Hsp70 expression in surviving cells indicating heat shock-related cell stress, while IVIS fluorescence showed a significant reduction of viable tumor cell numbers. Treated tumor centers displayed significant microscopic tumor damage with prominent signs of apoptosis, and major upregulation of cleaved/activated caspase-3-positive tumor cells. Serial sampling demonstrated substantial elevation of heat shock (Hsp70) response twelve hours after the treatment which was exhausted by twenty-four hours after treatment. Heat shock inhibitors Quercetin or KRIBB11 could synergistically amplify mEHT-induced tumor apoptosis in vitro. In conclusion, modulated electro-hyperthermia exerted a protective heat shock response as a clear sign of tumor cell stress. Exhaustion of the HSR manifested in caspase-dependent apoptotic tumor cell death and tissue damage of triple-negative breast cancer after mEHT monotherapy. Inhibiting the HSR synergistically increased the effect of mEHT. This finding has great translational potential.
Keywords: BALB/C mouse; heat-shock protein-70; isogenic mouse cancer; modulated electro-hyperthermia (mEHT); triple-negative breast cancer (TNBC).
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Digoxin-Mediated Inhibition of Potential Hypoxia-Related Angiogenic Repair in Modulated Electro-Hyperthermia (mEHT)-Treated Murine Triple-Negative Breast Cancer Model.ACS Pharmacol Transl Sci. 2024 Jan 9;7(2):456-466. doi: 10.1021/acsptsci.3c00296. eCollection 2024 Feb 9. ACS Pharmacol Transl Sci. 2024. PMID: 38357275 Free PMC article.
-
Heat shock factor 1 inhibition enhances the effects of modulated electro hyperthermia in a triple negative breast cancer mouse model.Sci Rep. 2024 Apr 8;14(1):8241. doi: 10.1038/s41598-024-57659-x. Sci Rep. 2024. PMID: 38589452 Free PMC article.
-
Modulated Electro-Hyperthermia Induces a Prominent Local Stress Response and Growth Inhibition in Mouse Breast Cancer Isografts.Cancers (Basel). 2021 Apr 6;13(7):1744. doi: 10.3390/cancers13071744. Cancers (Basel). 2021. PMID: 33917524 Free PMC article.
-
Targeting the heat shock response induced by modulated electro-hyperthermia (mEHT) in cancer.Biochim Biophys Acta Rev Cancer. 2024 Mar;1879(2):189069. doi: 10.1016/j.bbcan.2023.189069. Epub 2024 Jan 2. Biochim Biophys Acta Rev Cancer. 2024. PMID: 38176599 Review.
-
Modulated Electro-Hyperthermia-Induced Tumor Damage Mechanisms Revealed in Cancer Models.Int J Mol Sci. 2020 Aug 29;21(17):6270. doi: 10.3390/ijms21176270. Int J Mol Sci. 2020. PMID: 32872532 Free PMC article. Review.
Cited by
-
Effects of low-frequency ultrasound combined with microbubbles on breast cancer xenografts in nude mice.Glob Health Med. 2024 Aug 31;6(4):236-243. doi: 10.35772/ghm.2024.01037. Glob Health Med. 2024. PMID: 39219582 Free PMC article.
-
Desensitization of Capsaicin-Sensitive Afferents Accelerates Early Tumor Growth via Increased Vascular Leakage in a Murine Model of Triple Negative Breast Cancer.Front Oncol. 2021 Jul 14;11:685297. doi: 10.3389/fonc.2021.685297. eCollection 2021. Front Oncol. 2021. PMID: 34336669 Free PMC article.
-
Ivermectin Synergizes with Modulated Electro-hyperthermia and Improves Its Anticancer Effects in a Triple-Negative Breast Cancer Mouse Model.ACS Pharmacol Transl Sci. 2024 Jul 17;7(8):2496-2506. doi: 10.1021/acsptsci.4c00314. eCollection 2024 Aug 9. ACS Pharmacol Transl Sci. 2024. PMID: 39144564 Free PMC article.
-
External Basic Hyperthermia Devices for Preclinical Studies in Small Animals.Cancers (Basel). 2021 Sep 15;13(18):4628. doi: 10.3390/cancers13184628. Cancers (Basel). 2021. PMID: 34572855 Free PMC article. Review.
-
Digoxin-Mediated Inhibition of Potential Hypoxia-Related Angiogenic Repair in Modulated Electro-Hyperthermia (mEHT)-Treated Murine Triple-Negative Breast Cancer Model.ACS Pharmacol Transl Sci. 2024 Jan 9;7(2):456-466. doi: 10.1021/acsptsci.3c00296. eCollection 2024 Feb 9. ACS Pharmacol Transl Sci. 2024. PMID: 38357275 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

