Effects of New NSAID-CAI Hybrid Compounds in Inflammation and Lung Fibrosis
- PMID: 32927723
- PMCID: PMC7564963
- DOI: 10.3390/biom10091307
Effects of New NSAID-CAI Hybrid Compounds in Inflammation and Lung Fibrosis
Abstract
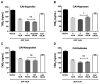
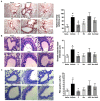
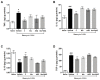
Pulmonary fibrosis is a severe lung disease with progressive worsening of dyspnea, characterized by chronic inflammation and remodeling of lung parenchyma. Carbonic anhydrases are a family of zinc-metallo-enzymes that catalyze the reversible interconversion of carbon-dioxide and water to bicarbonate and protons. Carbonic Anhydrase Inhibitor (CAI) exhibited anti-inflammatory effects in animals with permanent-middle-cerebral artery occlusion, arthritis and neuropathic pain. The pharmacological profile of a new class of hybrid compounds constituted by a CAI connected to a Nonsteroidal-Anti-Inflammatory Drug (NSAID) was studied in the modulation of inflammation and fibrosis. In-vitro tests were performed to assess their effects on cyclo-oxygenase enzyme (COX)-1 and COX-2, namely inhibition of platelet aggregation and thromboxane B2 production in the human-platelet-rich plasma, and reduction of Prostaglandin-E2 production in lipopolysaccharide-treated-RAW-264.7 macrophage cell line. The activity of compound 3, one of the most active, was studied in a model of bleomycin-induced lung fibrosis in C57BL/6 mice. The hybrid compounds showed a higher potency in inhibiting PGE2 production, but not in modifying the platelet aggregation and the TXB2 production in comparison to the reference molecules, indicating an increased activity in COX-2 inhibition. In the in-vivo murine model, the compound 3 was more effective in decreasing inflammation, lung stiffness and oxidative stress in comparison to the reference drugs given alone or in association. In conclusion, these CAI-NSAID hybrid compounds are promising new anti-inflammatory drugs for the treatment of lung chronic inflammatory diseases.
Keywords: CAI; COX-1; COX-2; NSAIDs; inflammation; pulmonary fibrosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Raghu G., Collard H.R., Egan J.J., Martinez F.J., Behr J., Brown K.K., Colby T.V., Cordier J.-F., Flaherty K.R., Lasky J.A., et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. - DOI - PMC - PubMed
-
- Raghu G., Rochwerg B., Zhang Y., Garcia C.A.C., Azuma A., Behr J., Brozek J.L., Collard H.R., Cunningham W., Homma S., et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2015;192:e3–e19. doi: 10.1164/rccm.201506-1063ST. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

