Isolation and Characterization of Two Novel Colorectal Cancer Cell Lines, Containing a Subpopulation with Potential Stem-Like Properties: Treatment Options by MYC/NMYC Inhibition
- PMID: 32927768
- PMCID: PMC7564713
- DOI: 10.3390/cancers12092582
Isolation and Characterization of Two Novel Colorectal Cancer Cell Lines, Containing a Subpopulation with Potential Stem-Like Properties: Treatment Options by MYC/NMYC Inhibition
Abstract
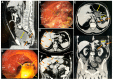
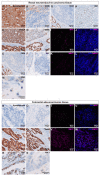
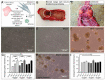
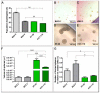
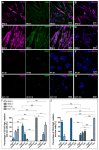
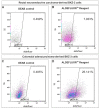
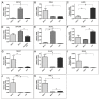
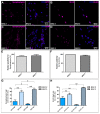
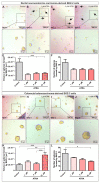
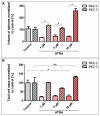
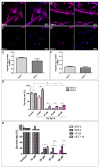
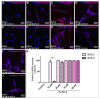
Cancer stem cells (CSC) are crucial mediators of cancer relapse. Here, we isolated two primary human colorectal cancer cell lines derived from a rectal neuroendocrine carcinoma (BKZ-2) and a colorectal adenocarcinoma (BKZ-3), both containing subpopulations with potential stem-like properties. Protein expression of CSC-markers prominin-1 and CD44 antigen was significantly higher for BKZ-2 and BKZ-3 in comparison to well-established colon carcinoma cell lines. High sphere-formation capacity further confirmed the existence of a subpopulation with potential stem-like phenotype. Epithelial-mesenchymal transition markers as well as immune checkpoint ligands were expressed more pronounced in BKZ-2. Both cell populations demonstrated N-myc proto-oncogene (NMYC) copy number gain. Myc proto-oncogene (MYC)/NMYC activity inhibitor all-trans retinoic acid (ATRA) significantly reduced the number of tumor spheres for both and the volume of BKZ-2 spheres. In contrast, the sphere volume of ATRA-treated BKZ-3 was increased, and only BKZ-2 cell proliferation was reduced in monolayer culture. Treatment with KJ-Pyr-9, a specific inhibitor of MYC/NMYC-myc-associated factor X interaction, decreased survival by the induction of apoptosis of both. In summary, here, we present the novel colorectal cancer cell lines BKZ-2 and BKZ-3 as promising cellular in vitro models for colorectal carcinomas and identify the MYC/NMYC molecular pathway involved in CSC-induced carcinogenesis with relevant therapeutic potential.
Keywords: ATRA; EMT; KJ-Pyr-9; MYC; NMYC; colorectal adenocarcinoma; colorectal cancer stem cells; rectal neuroendocrine carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The Diminishment of Novel Endometrial Carcinoma-Derived Stem-like Cells by Targeting Mitochondrial Bioenergetics and MYC.Int J Mol Sci. 2022 Feb 22;23(5):2426. doi: 10.3390/ijms23052426. Int J Mol Sci. 2022. PMID: 35269569 Free PMC article.
-
Novel Primary Human Cancer Stem-Like Cell Populations from Non-Small Cell Lung Cancer: Inhibition of Cell Survival by Targeting NF-κB and MYC Signaling.Cells. 2021 Apr 27;10(5):1024. doi: 10.3390/cells10051024. Cells. 2021. PMID: 33925297 Free PMC article.
-
Absence of functional CD44 hyaluronan receptor on human NMYC-amplified neuroblastoma cells.Cancer Res. 1997 Apr 1;57(7):1387-93. Cancer Res. 1997. PMID: 9102228
-
Triterpene Acid (3-O-p-Coumaroyltormentic Acid) Isolated From Aronia Extracts Inhibits Breast Cancer Stem Cell Formation through Downregulation of c-Myc Protein.Int J Mol Sci. 2018 Aug 26;19(9):2528. doi: 10.3390/ijms19092528. Int J Mol Sci. 2018. PMID: 30149665 Free PMC article. Review.
-
Stem cell models for genetically predisposed colon cancer.Oncol Lett. 2020 Nov;20(5):138. doi: 10.3892/ol.2020.11998. Epub 2020 Aug 20. Oncol Lett. 2020. PMID: 32934706 Free PMC article. Review.
Cited by
-
Efficient Radial-Shell Model for 3D Tumor Spheroid Dynamics with Radiotherapy.Cancers (Basel). 2023 Nov 29;15(23):5645. doi: 10.3390/cancers15235645. Cancers (Basel). 2023. PMID: 38067348 Free PMC article.
-
Nanopore Sequencing Reveals Global Transcriptome Signatures of Mitochondrial and Ribosomal Gene Expressions in Various Human Cancer Stem-like Cell Populations.Cancers (Basel). 2021 Mar 6;13(5):1136. doi: 10.3390/cancers13051136. Cancers (Basel). 2021. PMID: 33800955 Free PMC article.
-
The Diminishment of Novel Endometrial Carcinoma-Derived Stem-like Cells by Targeting Mitochondrial Bioenergetics and MYC.Int J Mol Sci. 2022 Feb 22;23(5):2426. doi: 10.3390/ijms23052426. Int J Mol Sci. 2022. PMID: 35269569 Free PMC article.
-
Resectable Colorectal Cancer: Current Perceptions on the Correlation of Recurrence Risk, Microbiota and Detection of Genetic Mutations in Liquid Biopsies.Cancers (Basel). 2021 Jul 14;13(14):3522. doi: 10.3390/cancers13143522. Cancers (Basel). 2021. PMID: 34298740 Free PMC article. Review.
-
Targeting NF-κB Signaling in Cancer Stem Cells: A Narrative Review.Biomedicines. 2022 Jan 25;10(2):261. doi: 10.3390/biomedicines10020261. Biomedicines. 2022. PMID: 35203471 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

