Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity
- PMID: 32928017
- PMCID: PMC7687032
- DOI: 10.1177/0271678X20951995
Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity
Abstract
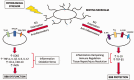
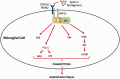
The blood-brain barrier (BBB) is a critical regulator of CNS homeostasis. It possesses physical and biochemical characteristics (i.e. tight junction protein complexes, transporters) that are necessary for the BBB to perform this physiological role. Microvascular endothelial cells require support from astrocytes, pericytes, microglia, neurons, and constituents of the extracellular matrix. This intricate relationship implies the existence of a neurovascular unit (NVU). NVU cellular components can be activated in disease and contribute to dynamic remodeling of the BBB. This is especially true of microglia, the resident immune cells of the brain, which polarize into distinct proinflammatory (M1) or anti-inflammatory (M2) phenotypes. Current data indicate that M1 pro-inflammatory microglia contribute to BBB dysfunction and vascular "leak", while M2 anti-inflammatory microglia play a protective role at the BBB. Understanding biological mechanisms involved in microglia activation provides a unique opportunity to develop novel treatment approaches for neurological diseases. In this review, we highlight characteristics of M1 proinflammatory and M2 anti-inflammatory microglia and describe how these distinct phenotypes modulate BBB physiology. Additionally, we outline the role of other NVU cell types in regulating microglial activation and highlight how microglia can be targeted for treatment of disease with a focus on ischemic stroke and Alzheimer's disease.
Keywords: Alzheimer’s disease; blood–brain barrier; inflammation; ischemic stroke; microglia; neurovascular unit; oxidative stress; paracellular permeability; tight junctions.
Conflict of interest statement
Figures

References
-
- Haseloff RF, Dithmer S, Winkler L, et al. Transmembrane proteins of the tight junctions at the blood-brain barrier: structural and functional aspects. Semin Cell Dev Biol 2015; 38: 16–25. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

