Time to adapt in the pandemic era: a prospective randomized non -inferiority study comparing time to intubate with and without the barrier box
- PMID: 32928122
- PMCID: PMC7488639
- DOI: 10.1186/s12871-020-01149-w
Time to adapt in the pandemic era: a prospective randomized non -inferiority study comparing time to intubate with and without the barrier box
Abstract
Background: The challenges posed by the spread of COVID-19 disease through aerosols have compelled anesthesiologists to modify their airway management practices. Devices such as barrier boxes are being considered as potential adjuncts to full PPE's to limit the aerosol spread. Usage of the barrier box raises concerns of delay in time to intubate (TTI). We designed our study to determine if using a barrier box with glidescope delays TTI within acceptable parameters to make relevant clinical conclusions.
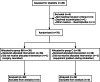
Methods: Seventy-eight patients were enrolled in this prospective non-inferiority controlled trial and were randomly allocated to either group C (without the barrier box) or the study group BB (using barrier box). The primary measured endpoint is time to intubate (TTI), which is defined as time taken from loss of twitches confirmed with a peripheral nerve stimulator to confirmation of end-tidal CO 2. 15 s was used as non-inferiority margin for the purpose of the study. We used an unpaired two-sample single-sided t-test to test our non- inferiority hypothesis (H 0: Mean TTI diff ≥15 s, H A: Mean TTI diff < 15 s). Secondary endpoints include the number of attempts at intubation, lowest oxygen saturation during induction, and the need for bag-mask ventilation.
Results: Mean TTI in group C was 42 s (CI 19.2 to 64.8) vs. 52.1 s (CI 26.1 to 78) in group BB. The difference in mean TTI was 10.1 s (CI -∞ to 14.9). We rejected the null hypothesis and concluded with 95% confidence that the difference of the mean TTI between the groups is less than < 15 s (95% CI -∞ to 14.9,p = 0.0461). Our induction times were comparable (67.7 vs. 65.9 s).100% of our patients were intubated on the first attempt in both groups. None of our patients needed rescue breaths.
Conclusions: We conclude that in patients with normal airway exam, scheduled for elective surgeries, our barrier box did not cause any clinically significant delay in TTI when airway manipulation is performed by well-trained providers. The study was retrospectively registered at clinicaltrials.gov (NCT04411056) on May 27, 2020.
Keywords: Airway management in COVID patients; Barrier box.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
The aerosol box for intubation in coronavirus disease 2019 patients: an in-situ simulation crossover study.Anaesthesia. 2020 Aug;75(8):1014-1021. doi: 10.1111/anae.15115. Epub 2020 Jun 1. Anaesthesia. 2020. PMID: 32397008 Free PMC article.
-
Measurement of airborne particle exposure during simulated tracheal intubation using various proposed aerosol containment devices during the COVID-19 pandemic.Anaesthesia. 2020 Dec;75(12):1587-1595. doi: 10.1111/anae.15188. Epub 2020 Jul 9. Anaesthesia. 2020. PMID: 32559315 Free PMC article.
-
Aerosol boxes and barrier enclosures for airway management in COVID-19 patients: a scoping review and narrative synthesis.Br J Anaesth. 2020 Dec;125(6):880-894. doi: 10.1016/j.bja.2020.08.038. Epub 2020 Sep 3. Br J Anaesth. 2020. PMID: 32977955 Free PMC article.
-
Aerosol Box 2.0: Adjustments and Improvements Made in Mexico for Intubating Patients During the Coronavirus Disease 2019 Pandemic.A A Pract. 2020 Jul;14(9):e01273. doi: 10.1213/XAA.0000000000001273. A A Pract. 2020. PMID: 32633929 Free PMC article.
-
Pediatric Airway Management in COVID-19 Patients: Consensus Guidelines From the Society for Pediatric Anesthesia's Pediatric Difficult Intubation Collaborative and the Canadian Pediatric Anesthesia Society.Anesth Analg. 2020 Jul;131(1):61-73. doi: 10.1213/ANE.0000000000004872. Anesth Analg. 2020. PMID: 32287142 Free PMC article. Review.
Cited by
-
The FEES box: A novel barrier to contain particles during aerosol-generating procedures.Am J Otolaryngol. 2021 May-Jun;42(3):102888. doi: 10.1016/j.amjoto.2020.102888. Epub 2021 Jan 4. Am J Otolaryngol. 2021. PMID: 33460980 Free PMC article.
-
Performance of Aerosol Boxes for Endotracheal Intubation during the COVID-19 Pandemic with Systematic Review.J Glob Infect Dis. 2023 Feb 28;15(1):6-12. doi: 10.4103/jgid.jgid_165_22. eCollection 2023 Jan-Mar. J Glob Infect Dis. 2023. PMID: 37090151 Free PMC article.
-
Macintosh laryngoscope and i-view™ and C-MAC® video laryngoscopes for tracheal intubation with an aerosol box: a randomized crossover manikin study.JA Clin Rep. 2021 Jun 26;7(1):52. doi: 10.1186/s40981-021-00455-7. JA Clin Rep. 2021. PMID: 34173923 Free PMC article.
-
Comparison of endotracheal intubation with Macintosh versus King Vision video laryngoscope using coronavirus disease 2019 barrier box on manikins: A randomized crossover study.Turk J Emerg Med. 2022 Jul 1;22(3):149-155. doi: 10.4103/2452-2473.348436. eCollection 2022 Jul-Sep. Turk J Emerg Med. 2022. PMID: 35936952 Free PMC article.
-
Intubation outcomes using the aerosol box during the COVID-19 pandemic: A prospective, observational study.Indian J Anaesth. 2021 Mar;65(3):221-228. doi: 10.4103/ija.IJA_1578_20. Epub 2021 Mar 13. Indian J Anaesth. 2021. PMID: 33776113 Free PMC article.
References
-
- COVID-19 and Anesthesia FAQ - Anesthesia Patient Safety Foundation [Internet]. [cited 2020 May 21]. Available from: https://www.apsf.org/covid-19-and-anesthesia-faq/.
-
- Should we use an “aerosol box” for intubation? • LITFL [Internet]. [cited 2020 May 6]. Available from: https://litfl.com/should-we-use-an-aerosol-box-for-intubation/.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical