Relative abundance of the Prevotella genus within the human gut microbiota of elderly volunteers determines the inter-individual responses to dietary supplementation with wheat bran arabinoxylan-oligosaccharides
- PMID: 32928123
- PMCID: PMC7490872
- DOI: 10.1186/s12866-020-01968-4
Relative abundance of the Prevotella genus within the human gut microbiota of elderly volunteers determines the inter-individual responses to dietary supplementation with wheat bran arabinoxylan-oligosaccharides
Abstract
Background: The human colon is colonised by a dense microbial community whose species composition and metabolism are linked to health and disease. The main energy sources for colonic bacteria are dietary polysaccharides and oligosaccharides. These play a major role in modulating gut microbial composition and metabolism, which in turn can impact on health outcomes.
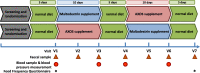
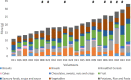
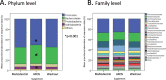
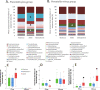
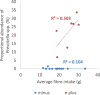
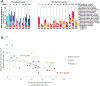
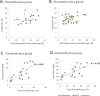
Results: We investigated the influence of wheat bran arabinoxylan oligosaccharides (AXOS) and maltodextrin supplements in modulating the composition of the colonic microbiota and metabolites in healthy adults over the age of 60. Male and female volunteers, (n = 21, mean BMI 25.2 ± 0.7 kg/m2) participated in the double-blind, cross over supplement study. Faecal samples were collected for analysis of microbiota, short chain fatty acids levels and calprotectin. Blood samples were collected to measure glucose, cholesterol and triglycerides levels. There was no change in these markers nor in calprotectin levels in response to the supplements. Both supplements were well-tolerated by the volunteers. Microbiota analysis across the whole volunteer cohort revealed a significant increase in the proportional abundance of faecal Bifidobacterium species (P ≤ 0.01) in response to AXOS, but not maltodextrin, supplementation. There was considerable inter-individual variation in the other bacterial taxa that responded, with a clear stratification of volunteers as either Prevotella-plus (n = 8; > 0.1% proportional abundance) or Prevotella-minus (n = 13; ≤0.1% proportional abundance) subjects founded on baseline sample profiles. There was a significant increase in the proportional abundance of both faecal Bifidobacterium (P ≤ 0.01) and Prevotella species (P ≤ 0.01) in Prevotella-plus volunteers during AXOS supplementation, while Prevotella and Bacteroides relative abundances showed an inverse relationship. Proportional abundance of 26 OTUs, including bifidobacteria and Anaerostipes hadrus, differed significantly between baseline samples of Prevotella-plus compared to Prevotella-minus individuals.
Conclusions: The wheat bran AXOS supplementation was bifidogenic and resulted in changes in human gut microbiota composition that depended on the initial microbiota profile, specifically the presence or absence of Prevotella spp. as a major component of the microbiota. Our data therefore suggest that initial profiling of individuals through gut microbiota analysis should be considered important when contemplating nutritional interventions that rely on prebiotics.
Trial registration: Clinical trial registration number: NCT02693782 . Registered 29 February 2016 - Retrospectively registered, https://clinicaltrials.gov/ct2/show/NCT02693782?term=NCT02693782&rank=1.
Keywords: Arabinoxylan oligosaccharides (AXOS); Bacteroides; Bifidobacteria; Butyrate; Diversity; Gut microbiota; Prevotella; Propionate; Short chain fatty acids; Wheatbran extract.
Conflict of interest statement
DB and VGC are employees of Cargill, who provided partial support for this work via a BBSRC CASE PhD studentship to WSFC. WSFC, AWW, JP, JW, SHD and HJF have no conflict of interest to declare. Cargill, Inc. is a producer of food ingredients. This does not alter our adherence to the journals policies on sharing data and materials.
Figures
Similar articles
-
Effect of wheat bran derived prebiotic supplementation on gastrointestinal transit, gut microbiota, and metabolic health: a randomized controlled trial in healthy adults with a slow gut transit.Gut Microbes. 2020 Nov 9;12(1):1704141. doi: 10.1080/19490976.2019.1704141. Epub 2020 Jan 25. Gut Microbes. 2020. PMID: 31983281 Free PMC article. Clinical Trial.
-
Arabinoxylan oligosaccharides and polyunsaturated fatty acid effects on gut microbiota and metabolic markers in overweight individuals with signs of metabolic syndrome: A randomized cross-over trial.Clin Nutr. 2020 Jan;39(1):67-79. doi: 10.1016/j.clnu.2019.01.012. Epub 2019 Feb 19. Clin Nutr. 2020. PMID: 30827722 Clinical Trial.
-
Gut microbiota modulation with long-chain corn bran arabinoxylan in adults with overweight and obesity is linked to an individualized temporal increase in fecal propionate.Microbiome. 2020 Aug 19;8(1):118. doi: 10.1186/s40168-020-00887-w. Microbiome. 2020. PMID: 32814582 Free PMC article.
-
Structure-dependent stimulation of gut bacteria by arabinoxylo-oligosaccharides (AXOS): a review.Gut Microbes. 2024 Jan-Dec;16(1):2430419. doi: 10.1080/19490976.2024.2430419. Epub 2024 Nov 29. Gut Microbes. 2024. PMID: 39611305 Free PMC article. Review.
-
Galacto-Oligosaccharides and the Elderly Gut: Implications for Immune Restoration and Health.Adv Nutr. 2024 Aug;15(8):100263. doi: 10.1016/j.advnut.2024.100263. Epub 2024 Jun 17. Adv Nutr. 2024. PMID: 38897384 Free PMC article. Review.
Cited by
-
Nutrition and Microbiome.Handb Exp Pharmacol. 2022;274:57-73. doi: 10.1007/164_2022_588. Handb Exp Pharmacol. 2022. PMID: 35434750 Review.
-
Effect of the administration of probiotics on the fecal microbiota of adult individuals.Food Sci Nutr. 2021 Oct 6;9(12):6471-6479. doi: 10.1002/fsn3.2547. eCollection 2021 Dec. Food Sci Nutr. 2021. PMID: 34925778 Free PMC article.
-
Diet-gut microbiome interaction and its impact on host blood glucose homeostasis: a series of nutritional n-of-1 trials.EBioMedicine. 2025 Jan;111:105483. doi: 10.1016/j.ebiom.2024.105483. Epub 2024 Dec 7. EBioMedicine. 2025. PMID: 39647263 Free PMC article. Clinical Trial.
-
Emerging probiotics: future therapeutics for human gut health.FEMS Microbiol Ecol. 2025 Jul 14;101(8):fiaf077. doi: 10.1093/femsec/fiaf077. FEMS Microbiol Ecol. 2025. PMID: 40711452 Free PMC article.
-
Assessing the safety of microbiome perturbations.Microb Genom. 2025 May;11(5):001405. doi: 10.1099/mgen.0.001405. Microb Genom. 2025. PMID: 40371892 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

