Tie2-mediated vascular remodeling by ferritin-based protein C nanoparticles confers antitumor and anti-metastatic activities
- PMID: 32928251
- PMCID: PMC7489044
- DOI: 10.1186/s13045-020-00952-9
Tie2-mediated vascular remodeling by ferritin-based protein C nanoparticles confers antitumor and anti-metastatic activities
Abstract
Background: Conventional therapeutic approaches for tumor angiogenesis, which are primarily focused on the inhibition of active angiogenesis to starve cancerous cells, target the vascular endothelial growth factor signaling pathway. This aggravates hypoxia within the tumor core and ultimately leads to increased tumor proliferation and metastasis. To overcome this limitation, we developed nanoparticles with antiseptic activity that target tumor vascular abnormalities.
Methods: Ferritin-based protein C nanoparticles (PCNs), known as TFG and TFMG, were generated and tested in Lewis lung carcinoma (LLC) allograft and MMTV-PyMT spontaneous breast cancer models. Immunohistochemical analysis was performed on tumor samples to evaluate the tumor vasculature. Western blot and permeability assays were used to explore the role and mechanism of the antitumor effects of PCNs in vivo. For knocking down proteins of interest, endothelial cells were transfected with siRNAs. Statistical analysis was performed using one-way ANOVA followed by post hoc Dunnett's multiple comparison test.
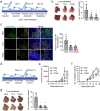
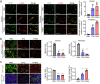
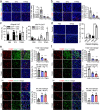
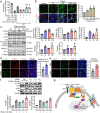
Results: PCNs significantly inhibited hypoxia and increased pericyte coverage, leading to the inhibition of tumor growth and metastasis, while increasing survival in LLC allograft and MMTV-PyMT spontaneous breast cancer models. The coadministration of cisplatin with PCNs induced a synergistic suppression of tumor growth by improving drug delivery as evidenced by increased blood prefusion and decreased vascular permeability. Moreover, PCNs altered the immune cell profiles within the tumor by increasing cytotoxic T cells and M1-like macrophages with antitumor activity. PCNs induced PAR-1/PAR-3 heterodimerization through EPCR occupation and PAR-1 activation, which resulted in Gα13-RhoA-mediated-Tie2 activation and stabilized vascular tight junctions via the Akt-FoxO3a signaling pathway.
Conclusions: Cancer treatment targeting the tumor vasculature by inducing antitumor immune responses and enhancing the delivery of a chemotherapeutic agent with PCNs resulted in tumor regression and may provide an effective therapeutic strategy.
Keywords: Antitumor immune response; EPCR; Ferritin-based protein C nanoparticles; Tie2; Vascular normalization.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
IFN-γ-mediated suppression of ANGPT2-Tie2 in endothelial cells facilitates tumor vascular normalization during immunotherapy.Front Immunol. 2025 Apr 30;16:1551322. doi: 10.3389/fimmu.2025.1551322. eCollection 2025. Front Immunol. 2025. PMID: 40370455 Free PMC article.
-
Early vascular deficits are correlated with delayed mammary tumorigenesis in the MMTV-PyMT transgenic mouse following genetic ablation of the NG2 proteoglycan.Breast Cancer Res. 2012 Apr 24;14(2):R67. doi: 10.1186/bcr3174. Breast Cancer Res. 2012. PMID: 22531600 Free PMC article.
-
Normalization of Tumor Vessels by Tie2 Activation and Ang2 Inhibition Enhances Drug Delivery and Produces a Favorable Tumor Microenvironment.Cancer Cell. 2016 Dec 12;30(6):953-967. doi: 10.1016/j.ccell.2016.10.018. Cancer Cell. 2016. PMID: 27960088
-
Functional significance of Tie2 signaling in the adult vasculature.Recent Prog Horm Res. 2004;59:51-71. doi: 10.1210/rp.59.1.51. Recent Prog Horm Res. 2004. PMID: 14749497 Review.
-
Tumor angiogenesis and accessibility: role of vascular endothelial growth factor.Semin Oncol. 2002 Dec;29(6 Suppl 16):3-9. doi: 10.1053/sonc.2002.37265. Semin Oncol. 2002. PMID: 12516032 Review.
Cited by
-
Precision nanomedicine: navigating the tumor microenvironment for enhanced cancer immunotherapy and targeted drug delivery.Mol Cancer. 2025 Jun 3;24(1):160. doi: 10.1186/s12943-025-02357-z. Mol Cancer. 2025. PMID: 40457437 Free PMC article. Review.
-
Nanotherapeutic Formulations for the Delivery of Cancer Antiangiogenics.Mol Pharm. 2025 May 5;22(5):2322-2349. doi: 10.1021/acs.molpharmaceut.4c00822. Epub 2025 Apr 4. Mol Pharm. 2025. PMID: 40184281 Free PMC article. Review.
-
Opportunities and Challenges of Nanoparticles in Digestive Tumours as Anti-Angiogenic Therapies.Front Oncol. 2022 Jan 10;11:789330. doi: 10.3389/fonc.2021.789330. eCollection 2021. Front Oncol. 2022. PMID: 35083147 Free PMC article. Review.
-
TME-Related Biomimetic Strategies Against Cancer.Int J Nanomedicine. 2024 Jan 4;19:109-135. doi: 10.2147/IJN.S441135. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38192633 Free PMC article. Review.
-
New strategies for lung cancer diagnosis and treatment: applications and advances in nanotechnology.Biomark Res. 2024 Nov 13;12(1):136. doi: 10.1186/s40364-024-00686-7. Biomark Res. 2024. PMID: 39533445 Free PMC article. Review.
References
-
- Kim C, Yang H, Fukushima Y, Saw PE, Lee J, Park JS, et al. Vascular RhoJ is an effective and selective target for tumor angiogenesis and vascular disruption. Cancer Cell. 2014;25(1):102–117. - PubMed
-
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–974. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

