Glutamate-glutamine homeostasis is perturbed in neurons and astrocytes derived from patient iPSC models of frontotemporal dementia
- PMID: 32928252
- PMCID: PMC7491073
- DOI: 10.1186/s13041-020-00658-6
Glutamate-glutamine homeostasis is perturbed in neurons and astrocytes derived from patient iPSC models of frontotemporal dementia
Abstract
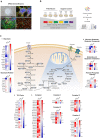
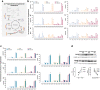
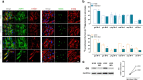
Frontotemporal dementia (FTD) is amongst the most prevalent early onset dementias and even though it is clinically, pathologically and genetically heterogeneous, a crucial involvement of metabolic perturbations in FTD pathology is being recognized. However, changes in metabolism at the cellular level, implicated in FTD and in neurodegeneration in general, are still poorly understood. Here we generate induced human pluripotent stem cells (hiPSCs) from patients carrying mutations in CHMP2B (FTD3) and isogenic controls generated via CRISPR/Cas9 gene editing with subsequent neuronal and glial differentiation and characterization. FTD3 neurons show a dysregulation of glutamate-glutamine related metabolic pathways mapped by 13C-labelling coupled to mass spectrometry. FTD3 astrocytes show increased uptake of glutamate whilst glutamate metabolism is largely maintained. Using quantitative proteomics and live-cell metabolic analyses, we elucidate molecular determinants and functional alterations of neuronal and glial energy metabolism in FTD3. Importantly, correction of the mutations rescues such pathological phenotypes. Notably, these findings implicate dysregulation of key enzymes crucial for glutamate-glutamine homeostasis in FTD3 pathogenesis which may underlie vulnerability to neurodegeneration. Neurons derived from human induced pluripotent stem cells (hiPSCs) of patients carrying mutations in CHMP2B (FTD3) display major metabolic alterations compared to CRISPR/Cas9 generated isogenic controls. Using quantitative proteomics, 13C-labelling coupled to mass spectrometry metabolic mapping and seahorse analyses, molecular determinants and functional alterations of neuronal and astrocytic energy metabolism in FTD3 were characterized. Our findings implicate dysregulation of glutamate-glutamine homeostasis in FTD3 pathogenesis. In addition, FTD3 neurons recapitulate glucose hypometabolism observed in FTD patient brains. The impaired mitochondria function found here is concordant with disturbed TCA cycle activity and decreased glycolysis in FTD3 neurons. FTD3 neuronal glutamine hypermetabolism is associated with up-regulation of PAG expression and, possibly, ROS production. Distinct compartments of glutamate metabolism can be suggested for the FTD3 neurons. Endogenous glutamate generated from glutamine via PAG may enter the TCA cycle via AAT (left side of neuron) while exogenous glutamate taken up from the extracellular space may be incorporated into the TCA cycle via GDH (right side of the neuron) FTD3 astrocytic glutamate uptake is upregulated whilst glutamate metabolism is largely maintained. Finally, pharmacological reversal of glutamate hypometabolism manifesting from decreased GDH expression should be explored as a novel therapeutic intervention for treating FTD3.
Keywords: CHMP2B; FTD3; GC-MS; GDH; GS; Glucose metabolism; Glutamate; Glutamine; PAG; iPSC-derived neuron.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Ahmed RM, et al. Amyotrophic lateral sclerosis and frontotemporal dementia: distinct and overlapping changes in eating behaviour and metabolism. Lancet Neurol. 2016;15:332–342. - PubMed
-
- Diehl-Schmid J, et al. Decline of cerebral glucose metabolism in frontotemporal dementia: a longitudinal 18F-FDG-PET-study. Neurobiol Aging. 2007;28:42–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

