The clinical potential of GDF15 as a "ready-to-feed indicator" for critically ill adults
- PMID: 32928255
- PMCID: PMC7488998
- DOI: 10.1186/s13054-020-03254-1
The clinical potential of GDF15 as a "ready-to-feed indicator" for critically ill adults
Abstract
Background: Circulating growth-differentiation factor-15 (GDF15), a cellular stress marker, abruptly increases during critical illness, but its later time course remains unclear. GDF15 physiologically controls oral intake by driving aversive responses to nutrition. Early parenteral nutrition (PN) in ICU patients has overall been shown not beneficial. We hypothesized that low GDF15 can identify patients who benefit from early PN, tolerate enteral nutrition (EN), and resume spontaneous oral intake.
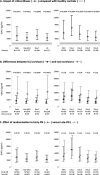
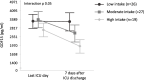
Methods: In secondary analyses of the EPaNIC-RCT on timing of PN initiation (early PN versus late PN) and the prospective observational DAS study, we documented the time course of circulating GDF15 in ICU (N = 1128) and 1 week post-ICU (N = 72), compared with healthy subjects (N = 65), and the impact hereon of randomization to early PN versus late PN in propensity score-matched groups (N = 564/group). Interaction between upon-admission GDF15 and randomization for its outcome effects was investigated (N = 4393). Finally, association between GDF15 and EN tolerance in ICU (N = 1383) and oral intake beyond ICU discharge (N = 72) was studied.
Results: GDF15 was elevated throughout ICU stay, similarly in early PN and late PN patients, and remained high beyond ICU discharge (p < 0.0001). Upon-admission GDF15 did not interact with randomization to early PN versus late PN for its outcome effects, but higher GDF15 independently related to worse outcomes (p ≤ 0.002). Lower GDF15 was only weakly related to gastrointestinal tolerance (p < 0.0001) and a steeper drop in GDF15 with more oral intake after ICU discharge (p = 0.05).
Conclusion: In critically ill patients, high GDF15 reflected poor prognosis and may contribute to aversive responses to nutrition. However, the potential of GDF15 as "ready-to-feed indicator" appears limited.
Trial registration: ClinicalTrials.gov , NCT00512122, registered 31 July 2007, https://www.clinicaltrials.gov/ct2/show/NCT00512122 (EPaNIC trial) and ISRCTN, ISRCTN 98806770, registered 11 November 2014, http://www.isrctn.com/ISRCTN98806770 (DAS trial).
Keywords: Critical illness; Feeding intolerance; GDF15; Outcome; Parenteral nutrition.
Conflict of interest statement
None of the authors has any conflict of interest to report.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 1151018N/Research Foundation-Flanders (FWO)/International
- 11W9317N/Research Foundation-Flanders (FWO)/International
- 1832817N/Research Foundation-Flanders (FWO)/International
- METH14/06/Methusalem program of the Flemish government/International
- METH14/06/Methusalem program of the Flemish government/International
- AdvG-2012-321670/FP7 Ideas: European Research Council ()/International
- 2017-785809/H2020 European Research Council/International
- Postdoctoral research fellowship/Universitaire Ziekenhuizen Leuven, KU Leuven/International
- C24/17/070/KU Leuven/International
- 24/17/070/KU Leuven/International
- STG-16-00253/KU Leuven/International
LinkOut - more resources
Full Text Sources
Medical
Research Materials

