Beta-alanine supplementation in patients with COPD receiving non-linear periodised exercise training or neuromuscular electrical stimulation: protocol of two randomised, double-blind, placebo-controlled trials
- PMID: 32928863
- PMCID: PMC7488791
- DOI: 10.1136/bmjopen-2020-038836
Beta-alanine supplementation in patients with COPD receiving non-linear periodised exercise training or neuromuscular electrical stimulation: protocol of two randomised, double-blind, placebo-controlled trials
Abstract
Introduction: Exercise intolerance is common in patients with chronic obstructive pulmonary disease (COPD) and, although multifactorial, it is largely caused by lower-limb muscle dysfunction. Research has shown that patients with severe to very severe COPD have significantly lower levels of muscle carnosine, which acts as a pH buffer and antioxidant. Beta-alanine (BA) supplementation has been shown to consistently elevate muscle carnosine in a variety of populations and may therefore improve exercise tolerance and lower-limb muscle function. The primary objective of the current studies is to assess the beneficial effects of BA supplementation in enhancing exercise tolerance on top of two types of exercise training (non-linear periodised exercise (NLPE) training or neuromuscular electrical stimulation (NMES)) in patients with COPD.
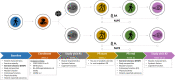
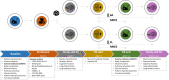
Methods and analysis: Two randomised, double-blind, placebo-controlled trials have been designed. Patients will routinely receive either NLPE (BASE-TRAIN trial) or NMES (BASE-ELECTRIC trial) as part of standard exercise-based care during their 8-to-10 week pulmonary rehabilitation (PR) programme. A total of 222 patients with COPD (2×77 = 154 patients in the BASE-TRAIN trial and 2×34 = 68 patients in the BASE-ELECTRIC trial) will be recruited from two specialised PR centres in The Netherlands. For study purposes, patients will receive 3.2 g of oral BA supplementation or placebo per day. Exercise tolerance is the primary outcome, which will be assessed using the endurance shuttle walk test (BASE-TRAIN) or the constant work rate cycle test (BASE-ELECTRIC). Furthermore, quadriceps muscle strength and endurance, cognitive function, carnosine levels (in muscle), BA levels (in blood and muscle), markers of oxidative stress and inflammation (in blood, muscles and lungs), physical activity and quality of life will be measured.
Ethics and dissemination: Both trials were approved by CMO Regio Arnhem-Nijmegen, The Netherlands (NL70781.091.19. and NL68757.091.19).
Trial registration number: NTR8427 (BASE-TRAIN) and NTR8419 (BASE-ELECTRIC).
Keywords: chronic airways disease; nutrition & dietetics; rehabilitation medicine; respiratory medicine (see thoracic medicine).
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: FMEF reports research grants from AstraZeneca and Novartis, not related to the current projects, and personal fees for consultancies and lectures from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis and TEVA. EBdV reports personal fees for consultancies and lectures from Boehringer Ingelheim, Chiesi, Mylan, Novartis, Vivisol and TEVA, not related to the current projects. FNS reports speakers fees from AstraZeneca, Chiesi, Menarini, Mundipharma and Novartis; reports consultancy fees from GSK; and reports service on an advisory board for AstraZeneca, Chiesi, GSK and Novartis, all outside the submitted work. MH reports research grants from Bastide Medical, not related to the current project; personal fees from AstraZeneca for participation to scientific lectures; financial support for congress participation from SOS Oxygène, Eole Santé, Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca; and hospitalities during local scientific meetings from ALK-Abelló, Actelion Pharmaceuticals France, Vifor Fresenius Medical Care Renal Pharma, Sanofi Aventis France, Novartis Pharma, LVL Medical Sud, Chiesi, SOS Oxygene Mediterranee. MAS reports a research grant from Netherlands Lung Foundation (grant number 5.1.18.232) for the described BASE-TRAIN and BASE-ELECTRIC study. Moreover, MAS reports other grants from Netherlands Lung Foundation, grants and personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, and a grant from Stichting Astma Bestrijding, all outside the submitted work.
Figures
Similar articles
-
Efficacy of 12 weeks oral beta-alanine supplementation in patients with chronic obstructive pulmonary disease: a double-blind, randomized, placebo-controlled trial.J Cachexia Sarcopenia Muscle. 2022 Oct;13(5):2361-2372. doi: 10.1002/jcsm.13048. Epub 2022 Aug 17. J Cachexia Sarcopenia Muscle. 2022. PMID: 35977911 Free PMC article. Clinical Trial.
-
Neuromuscular electrical stimulation to improve exercise capacity in patients with severe COPD: a randomised double-blind, placebo-controlled trial.Lancet Respir Med. 2016 Jan;4(1):27-36. doi: 10.1016/S2213-2600(15)00503-2. Epub 2015 Dec 15. Lancet Respir Med. 2016. PMID: 26701362 Clinical Trial.
-
Creatine supplementation and physical training in patients with COPD: a double blind, placebo-controlled study.Int J Chron Obstruct Pulmon Dis. 2006;1(4):445-53. doi: 10.2147/copd.2006.1.4.445. Int J Chron Obstruct Pulmon Dis. 2006. PMID: 18044100 Free PMC article. Clinical Trial.
-
Effectiveness of neuromuscular electrical stimulation for the rehabilitation of moderate-to-severe COPD: a meta-analysis.Int J Chron Obstruct Pulmon Dis. 2016 Nov 28;11:2965-2975. doi: 10.2147/COPD.S120555. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27932876 Free PMC article. Review.
-
Effects of neuromuscular electrical stimulation on exercise capacity and quality of life in COPD patients: a systematic review and meta-analysis.Biosci Rep. 2020 May 29;40(5):BSR20191912. doi: 10.1042/BSR20191912. Biosci Rep. 2020. PMID: 32368783 Free PMC article.
Cited by
-
Efficacy of 12 weeks oral beta-alanine supplementation in patients with chronic obstructive pulmonary disease: a double-blind, randomized, placebo-controlled trial.J Cachexia Sarcopenia Muscle. 2022 Oct;13(5):2361-2372. doi: 10.1002/jcsm.13048. Epub 2022 Aug 17. J Cachexia Sarcopenia Muscle. 2022. PMID: 35977911 Free PMC article. Clinical Trial.
-
The gut microbiota from maintenance hemodialysis patients with sarcopenia influences muscle function in mice.Front Cell Infect Microbiol. 2023 Sep 12;13:1225991. doi: 10.3389/fcimb.2023.1225991. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37771694 Free PMC article.
-
Practical Guidelines by the Andalusian Group for Nutrition Reflection and Investigation (GARIN) on Nutritional Management of Patients with Chronic Obstructive Pulmonary Disease: A Review.Nutrients. 2024 Sep 14;16(18):3105. doi: 10.3390/nu16183105. Nutrients. 2024. PMID: 39339705 Free PMC article.
-
Inositol possesses antifibrotic activity and mitigates pulmonary fibrosis.Respir Res. 2023 May 16;24(1):132. doi: 10.1186/s12931-023-02421-6. Respir Res. 2023. PMID: 37194070 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
