Establishment of a relationship between blastomere geometry and YAP localisation during compaction
- PMID: 32928909
- PMCID: PMC7561472
- DOI: 10.1242/dev.189449
Establishment of a relationship between blastomere geometry and YAP localisation during compaction
Abstract
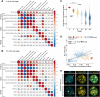
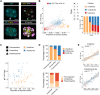
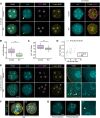
Precise patterning within the three-dimensional context of tissues, organs and embryos implies that cells can sense their relative position. During preimplantation development, outside and inside cells rely on apicobasal polarity and the Hippo pathway to choose their fate. Despite recent findings suggesting that mechanosensing might be central to this process, the relationship between blastomere geometry (i.e. shape and position) and the Hippo pathway effector YAP remains unknown. We used a highly quantitative approach to analyse information on the geometry and YAP localisation of individual blastomeres of mouse and human embryos. We identified the proportion of exposed cell surface area as most closely correlating with the nuclear localisation of YAP. To test this relationship, we developed several hydrogel-based approaches to alter blastomere geometry in cultured embryos. Unbiased clustering analyses of blastomeres from such embryos revealed that this relationship emerged during compaction. Our results therefore pinpoint the time during early embryogenesis when cells acquire the ability to sense changes in geometry and provide a new framework for how cells might integrate signals from different membrane domains to assess their relative position within the embryo.
Keywords: Biocompatible polymers; Compaction; Hippo signalling; Human embryo.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
-
- Couzens A. L., Knight J. D. R., Kean M. J., Teo G., Weiss A., Dunham W. H., Lin Z.-Y., Bagshaw R. D., Sicheri F., Pawson T. et al. (2013). Protein interaction network of the mammalian hippo pathway reveals mechanisms of kinase-phosphatase interactions. Sci. Signal. 6, rs15-rs15 10.1126/scisignal.2004712 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

