The solution structure of the complement deregulator FHR5 reveals a compact dimer and provides new insights into CFHR5 nephropathy
- PMID: 32928961
- PMCID: PMC7705313
- DOI: 10.1074/jbc.RA120.015132
The solution structure of the complement deregulator FHR5 reveals a compact dimer and provides new insights into CFHR5 nephropathy
Abstract
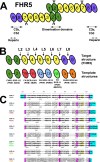
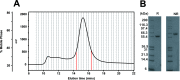
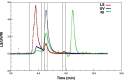
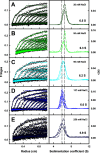
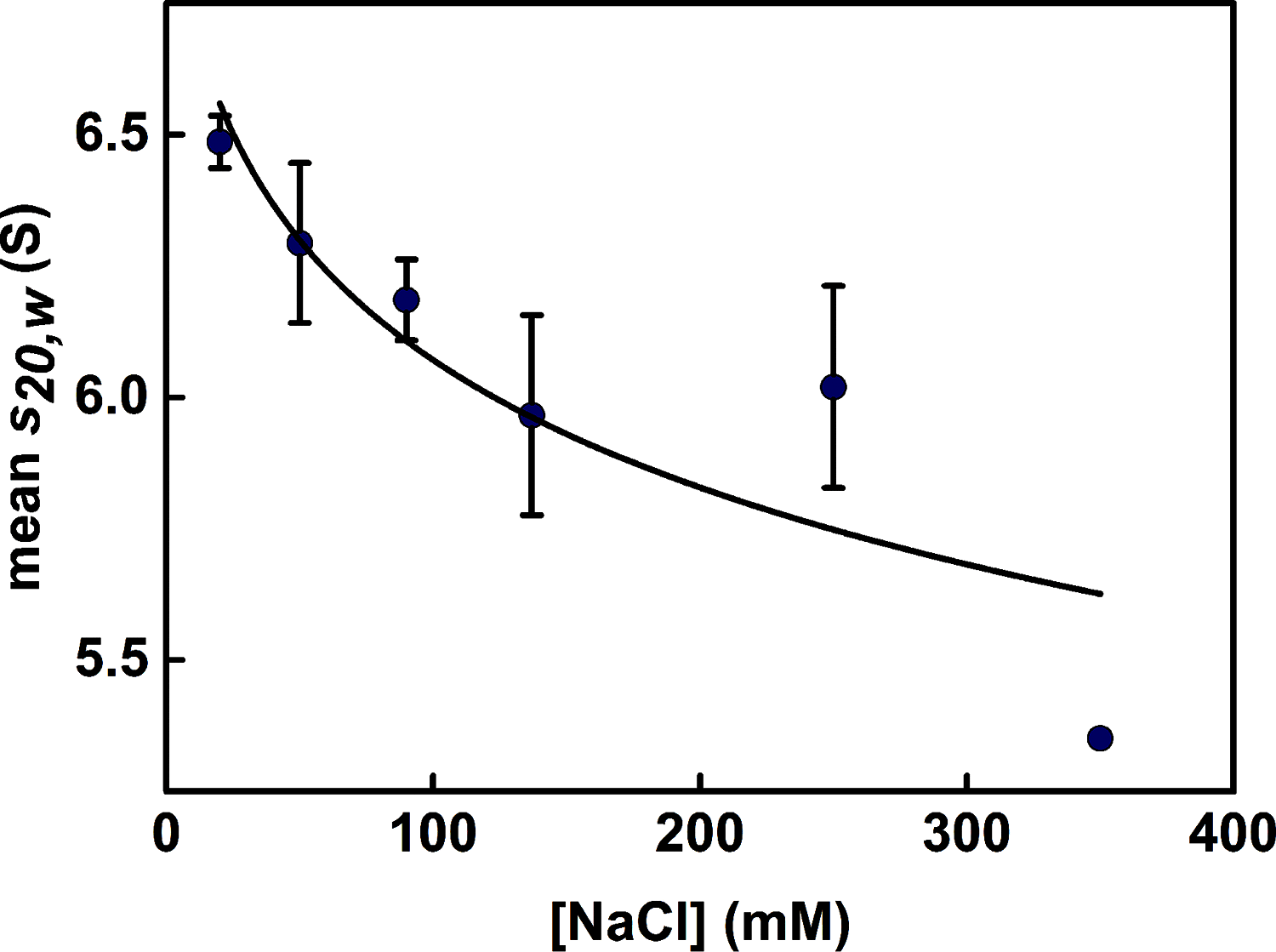
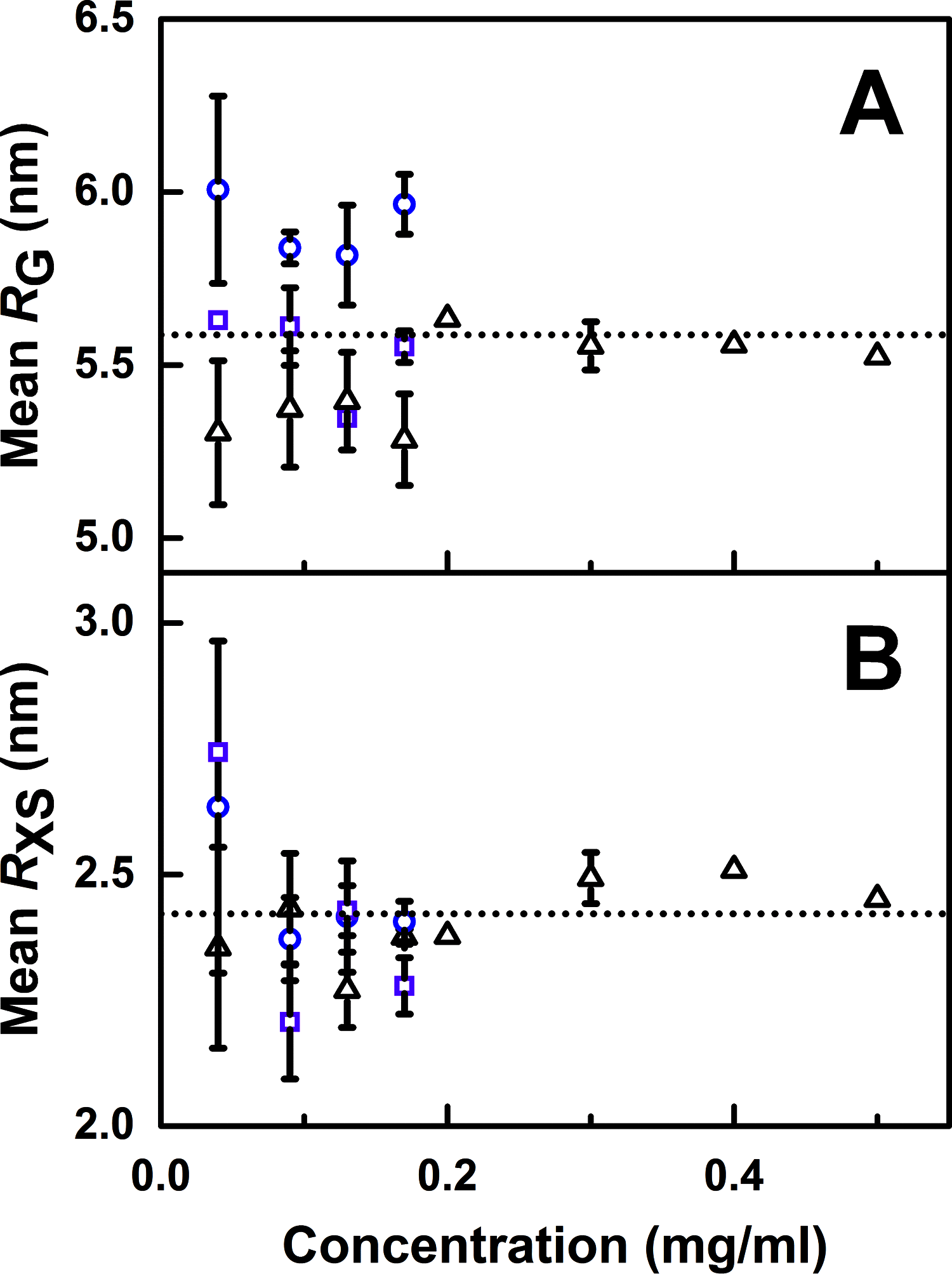
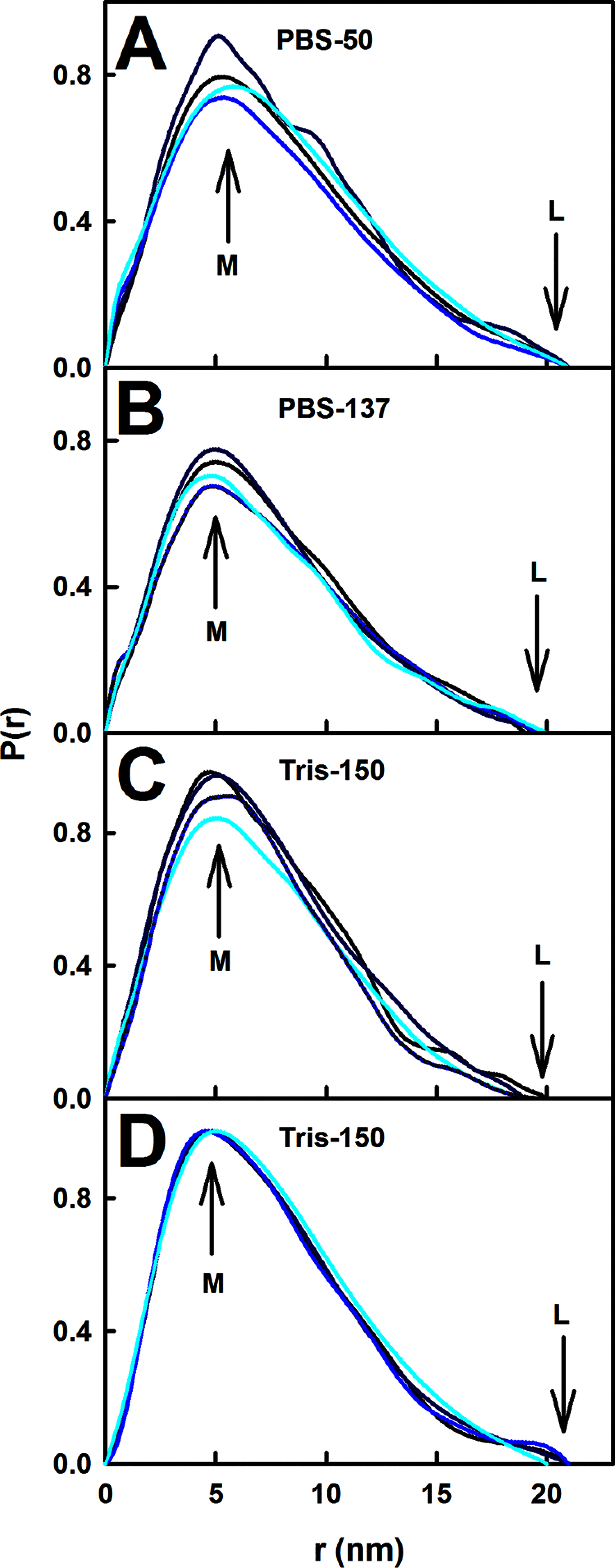
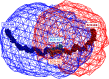
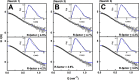
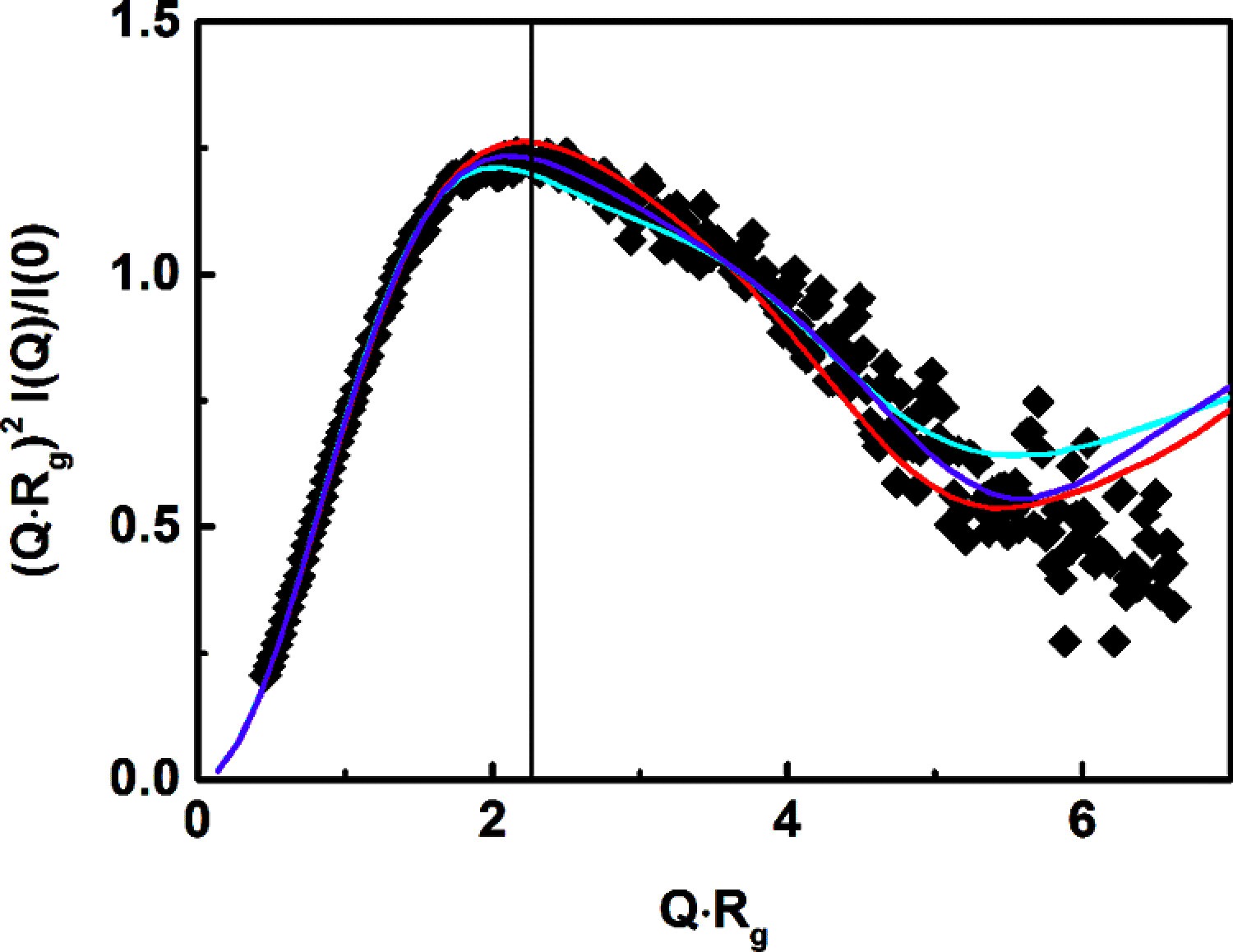
The human complement Factor H-related 5 protein (FHR5) antagonizes the main circulating complement regulator Factor H, resulting in the deregulation of complement activation. FHR5 normally contains nine short complement regulator (SCR) domains, but a FHR5 mutant has been identified with a duplicated N-terminal SCR-1/2 domain pair that causes CFHR5 nephropathy. To understand how this duplication causes disease, we characterized the solution structure of native FHR5 by analytical ultracentrifugation and small-angle X-ray scattering. Sedimentation velocity and X-ray scattering indicated that FHR5 was dimeric, with a radius of gyration (Rg ) of 5.5 ± 0.2 nm and a maximum protein length of 20 nm for its 18 domains. This result indicated that FHR5 was even more compact than the main regulator Factor H, which showed an overall length of 26-29 nm for its 20 SCR domains. Atomistic modeling for FHR5 generated a library of 250,000 physically realistic trial arrangements of SCR domains for scattering curve fits. Only compact domain structures in this library fit well to the scattering data, and these structures readily accommodated the extra SCR-1/2 domain pair present in CFHR5 nephropathy. This model indicated that mutant FHR5 can form oligomers that possess additional binding sites for C3b in FHR5. We conclude that the deregulation of complement regulation by the FHR5 mutant can be rationalized by the enhanced binding of FHR5 oligomers to C3b deposited on host cell surfaces. Our FHR5 structures thus explained key features of the mechanism and pathology of CFHR5 nephropathy.
Keywords: FHR5; Monte Carlo simulations; analytical ultracentrifugation; atomistic modeling; complement; molecular dynamics; molecular modeling; small-angle X-ray scattering (SAXS); small-angle X-ray scattering analytical ultracentrifugation.
© 2020 Kadkhodayi-Kholghi et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- McRae J. L., Duthy T. G., Griggs K. M., Ormsby R. J., Cowan P. J., Cromer B. A., McKinstry W. J., Parker M. W., Murphy B. F., and Gordon D. L. (2005) Human factor H-related protein 5 has cofactor activity, inhibits C3 convertase activity, binds heparin and C-reactive protein, and associates with lipoprotein. J. Immunol. 174, 6250–6256 10.4049/jimmunol.174.10.6250 - DOI - PubMed
-
- Soares D., and Barlow P. N. (2005) Complement control protein modules in the regulators of complement activators. In Structural Biology of the Complement System (Morikis D., and Lambris J. D., eds) pp. 19–62, Taylor & Francis, Boca Raton, FL
-
- Goicoechea de Jorge E., Caesar J. J., Malik T. H., Patel M., Colledge M., Johnson S., Hakobyan S., Morgan B. P., Harris C. L., Pickering M. C., and Lea S. M. (2013) Dimerization of complement factor H-related proteins modulates complement activation in vivo. Proc. Natl. Acad. Sci. U. S. A. 110, 4685–4690 10.1073/pnas.1219260110 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

