Role of peripheral sensory neuron mu-opioid receptors in nociceptive, inflammatory, and neuropathic pain
- PMID: 32928995
- PMCID: PMC8692009
- DOI: 10.1136/rapm-2020-101779
Role of peripheral sensory neuron mu-opioid receptors in nociceptive, inflammatory, and neuropathic pain
Abstract
Background and objective: The role of peripheral mu-opioid receptors (MOPs) in chronic pain conditions is not well understood. Here, we used a combination of mouse genetics, behavioral assays, and pharmacologic interventions to investigate the contribution of primary afferent MOPs to nociceptive, inflammatory, and neuropathic pain, as well as to opioid analgesia.
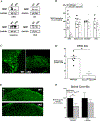
Methods: We generated conditional knockout mice in which MOPs were selectively deleted in primary sensory neurons. Inflammatory and neuropathic pain states were induced in mutant and control wild-type mice and their behavioral responses to noxious stimuli were compared. Gross motor function was also evaluated. Immunohistochemistry was used to assess MOP expression in the dorsal root ganglia, periaqueductal gray, and small intestine. The effects of MOP agonists DALDA (dermorphin [D-Arg2, Lys4] (1-4) amide) and morphine were evaluated in pain behavior assays, and their effects on neuronal physiology in the dorsal root ganglia were evaluated in whole-cell patch-clamp recordings.
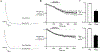
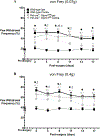
Results: Conditional MOP knockouts and control mice exhibited similar behavioral responses to acute nociceptive stimuli and developed similar inflammation-induced hypersensitivity. Unilateral nerve injury in animals lacking peripheral MOPs induced enhanced, bilateral mechanical allodynia. Subcutaneously administered DALDA was unable to decrease the hypersensitivity induced by inflammation and nerve injury in MOP knockout animals, and morphine's antinociceptive effects were significantly attenuated in the absence of peripheral MOPs.
Conclusion: MOPs in primary sensory neurons contribute to the modulation of neuropathic pain behavior and opioid analgesia. Our observations highlight the clinical potential of peripherally acting opioid agonists in the management of inflammatory and neuropathic pain.
Keywords: analgesics; chronic pain; drug-related side effects and adverse reactions; neuralgia; opioid; pain management.
© American Society of Regional Anesthesia & Pain Medicine 2020. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: YG serves as the executive editor for basic science for the Regional Anesthesia & Pain Medicine journal. SR is a consultant for Allergan, Averitas Pharma, Bayer, and Lexicon Pharmaceuticals, and has consulted for Aptinyx, Heron Therapeutics, and Insys Therapeutics. YG and SR are principal and co-investigators in a research grant from Medtronic.
Figures




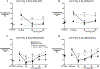
Similar articles
-
Activation of Peripheral μ-opioid Receptors by Dermorphin [D-Arg2, Lys4] (1-4) Amide Leads to Modality-preferred Inhibition of Neuropathic Pain.Anesthesiology. 2016 Mar;124(3):706-20. doi: 10.1097/ALN.0000000000000993. Anesthesiology. 2016. PMID: 26756519 Free PMC article.
-
Mu opioid receptors on primary afferent nav1.8 neurons contribute to opiate-induced analgesia: insight from conditional knockout mice.PLoS One. 2013 Sep 12;8(9):e74706. doi: 10.1371/journal.pone.0074706. eCollection 2013. PLoS One. 2013. PMID: 24069332 Free PMC article.
-
Mu and delta opioid receptors play opposite nociceptive and behavioural roles on nerve-injured mice.Br J Pharmacol. 2020 Mar;177(5):1187-1205. doi: 10.1111/bph.14911. Epub 2020 Feb 10. Br J Pharmacol. 2020. PMID: 31655493 Free PMC article.
-
The plasticity of the association between mu-opioid receptor and glutamate ionotropic receptor N in opioid analgesic tolerance and neuropathic pain.Eur J Pharmacol. 2013 Sep 15;716(1-3):94-105. doi: 10.1016/j.ejphar.2013.01.066. Epub 2013 Mar 13. Eur J Pharmacol. 2013. PMID: 23499699 Review.
-
Opioids in chronic pain.Eur J Pharmacol. 2001 Oct 19;429(1-3):79-91. doi: 10.1016/s0014-2999(01)01308-5. Eur J Pharmacol. 2001. PMID: 11698029 Review.
Cited by
-
Characterizing a new rat model of chronic pain after spine surgery.Bone Res. 2025 Mar 12;13(1):34. doi: 10.1038/s41413-025-00408-1. Bone Res. 2025. PMID: 40074742 Free PMC article.
-
Role of primary sensory neurone cannabinoid type-1 receptors in pain and the analgesic effects of the peripherally acting agonist CB-13 in mice.Br J Anaesth. 2022 Jan;128(1):159-173. doi: 10.1016/j.bja.2021.10.020. Epub 2021 Nov 26. Br J Anaesth. 2022. PMID: 34844727 Free PMC article.
-
Neuroprotective effects of naltrexone in a mouse model of post-traumatic seizures.Sci Rep. 2024 Jun 12;14(1):13507. doi: 10.1038/s41598-024-63942-8. Sci Rep. 2024. PMID: 38867062 Free PMC article.
-
Targeting sensory neuron GPCRs for peripheral neuropathic pain.Trends Pharmacol Sci. 2023 Dec;44(12):1009-1027. doi: 10.1016/j.tips.2023.10.003. Epub 2023 Nov 11. Trends Pharmacol Sci. 2023. PMID: 37977131 Free PMC article. Review.
-
Peripherally restricted cannabinoid and mu-opioid receptor agonists synergistically attenuate neuropathic mechanical hypersensitivity in mice.Pain. 2024 Nov 1;165(11):2563-2577. doi: 10.1097/j.pain.0000000000003278. Epub 2024 May 30. Pain. 2024. PMID: 38815196
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
