Microglia depletion exacerbates demyelination and impairs remyelination in a neurotropic coronavirus infection
- PMID: 32929007
- PMCID: PMC7533697
- DOI: 10.1073/pnas.2007814117
Microglia depletion exacerbates demyelination and impairs remyelination in a neurotropic coronavirus infection
Abstract
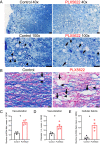
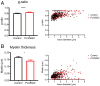
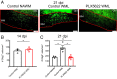
Microglia are considered both pathogenic and protective during recovery from demyelination, but their precise role remains ill defined. Here, using an inhibitor of colony stimulating factor 1 receptor (CSF1R), PLX5622, and mice infected with a neurotropic coronavirus (mouse hepatitis virus [MHV], strain JHMV), we show that depletion of microglia during the time of JHMV clearance resulted in impaired myelin repair and prolonged clinical disease without affecting the kinetics of virus clearance. Microglia were required only during the early stages of remyelination. Notably, large deposits of extracellular vesiculated myelin and cellular debris were detected in the spinal cords of PLX5622-treated and not control mice, which correlated with decreased numbers of oligodendrocytes in demyelinating lesions in drug-treated mice. Furthermore, gene expression analyses demonstrated differential expression of genes involved in myelin debris clearance, lipid and cholesterol recycling, and promotion of oligodendrocyte function. The results also demonstrate that microglial functions affected by depletion could not be compensated by infiltrating macrophages. Together, these results demonstrate that microglia play key roles in debris clearance and in the initiation of remyelination following infection with a neurotropic coronavirus but are not necessary during later stages of remyelination.
Keywords: coronavirus; microglia; neuroinflammation; remyelination; virus-induced demyelination.
Conflict of interest statement
The authors declare no competing interest.
Figures





References
-
- Major E. O., Yousry T. A., Clifford D. B., Pathogenesis of progressive multifocal leukoencephalopathy and risks associated with treatments for multiple sclerosis: A decade of lessons learned. Lancet Neurol. 17, 467–480 (2018). - PubMed
-
- Ozden S., Seilhean D., Gessain A., Hauw J.-J., Gout O., Severe demyelinating myelopathy with low human T cell lymphotropic virus type 1 expression after transfusion in an immunosuppressed patient. Clin. Infect. Dis. 34, 855–860 (2002). - PubMed
-
- Hall W. W., Choppin P. W., Measles-virus proteins in the brain tissue of patients with subacute sclerosing panencephalitis: Absence of the M protein. N. Engl. J. Med. 304, 1152–1155 (1981). - PubMed
-
- Sundqvist E. et al. ., Epstein-Barr virus and multiple sclerosis: Interaction with HLA. Genes Immun. 13, 14–20 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

