Regulation of titin-based cardiac stiffness by unfolded domain oxidation (UnDOx)
- PMID: 32929035
- PMCID: PMC7533878
- DOI: 10.1073/pnas.2004900117
Regulation of titin-based cardiac stiffness by unfolded domain oxidation (UnDOx)
Abstract
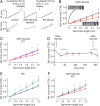
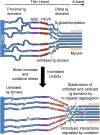
The relationship between oxidative stress and cardiac stiffness is thought to involve modifications to the giant muscle protein titin, which in turn can determine the progression of heart disease. In vitro studies have shown that S-glutathionylation and disulfide bonding of titin fragments could alter the elastic properties of titin; however, whether and where titin becomes oxidized in vivo is less certain. Here we demonstrate, using multiple models of oxidative stress in conjunction with mechanical loading, that immunoglobulin domains preferentially from the distal titin spring region become oxidized in vivo through the mechanism of unfolded domain oxidation (UnDOx). Via oxidation type-specific modification of titin, UnDOx modulates human cardiomyocyte passive force bidirectionally. UnDOx also enhances titin phosphorylation and, importantly, promotes nonconstitutive folding and aggregation of unfolded domains. We propose a mechanism whereby UnDOx enables the controlled homotypic interactions within the distal titin spring to stabilize this segment and regulate myocardial passive stiffness.
Keywords: mechanics; myocardial stiffness; oxidative stress; proteomics; single-molecule measurements.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

