Activation of Wnt signaling by amniotic fluid stem cell-derived extracellular vesicles attenuates intestinal injury in experimental necrotizing enterocolitis
- PMID: 32929076
- PMCID: PMC7490270
- DOI: 10.1038/s41419-020-02964-2
Activation of Wnt signaling by amniotic fluid stem cell-derived extracellular vesicles attenuates intestinal injury in experimental necrotizing enterocolitis
Abstract
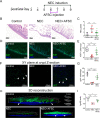
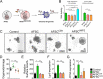
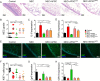
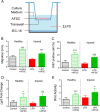
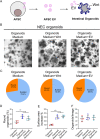
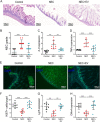
Necrotizing enterocolitis (NEC) is a devastating intestinal disease primarily affecting preterm neonates and causing high morbidity, high mortality, and huge costs for the family and society. The treatment and the outcome of the disease have not changed in recent decades. Emerging evidence has shown that stimulating the Wnt/β-catenin pathway and enhancing intestinal regeneration are beneficial in experimental NEC, and that they could potentially be used as a novel treatment. Amniotic fluid stem cells (AFSC) and AFSC-derived extracellular vesicles (EV) can be used to improve intestinal injury in experimental NEC. However, the mechanisms by which they affect the Wnt/β-catenin pathway and intestinal regeneration are unknown. In our current study, we demonstrated that AFSC and EV attenuate NEC intestinal injury by activating the Wnt signaling pathway. AFSC and EV stimulate intestinal recovery from NEC by increasing cellular proliferation, reducing inflammation and ultimately regenerating a normal intestinal epithelium. EV administration has a rescuing effect on intestinal injury when given during NEC induction; however, it failed to prevent injury when given prior to NEC induction. AFSC-derived EV administration is thus a potential emergent novel treatment strategy for NEC.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Yee WH, et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. 2012;129:e298–e304. - PubMed
-
- Pierro A. The surgical management of necrotising enterocolitis. Early Hum. Dev. 2005;81:79–85. - PubMed
-
- Thyoka M, et al. Advanced necrotizing enterocolitis part 1: mortality. Eur. J. Pediatr. Surg. 2012;22:8–12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

