Ketamine modulates fronto-striatal circuitry in depressed and healthy individuals
- PMID: 32929215
- PMCID: PMC8462973
- DOI: 10.1038/s41380-020-00878-1
Ketamine modulates fronto-striatal circuitry in depressed and healthy individuals
Abstract
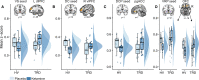
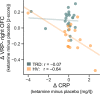
Ketamine improves motivation-related symptoms in depression but simultaneously elicits similar symptoms in healthy individuals, suggesting that it might have different effects in health and disease. This study examined whether ketamine affects the brain's fronto-striatal system, which is known to drive motivational behavior. The study also assessed whether inflammatory mechanisms-which are known to influence neural and behavioral motivational processes-might underlie some of these changes. These questions were explored in the context of a double-blind, placebo-controlled, crossover trial of ketamine in 33 individuals with treatment-resistant major depressive disorder (TRD) and 25 healthy volunteers (HVs). Resting-state functional magnetic resonance imaging (rsfMRI) was acquired 2 days post-ketamine (final sample: TRD n = 27, HV n = 19) and post-placebo (final sample: TRD n = 25, HV n = 18) infusions and was used to probe fronto-striatal circuitry with striatal seed-based functional connectivity. Ketamine increased fronto-striatal functional connectivity in TRD participants toward levels observed in HVs while shifting the connectivity profile in HVs toward a state similar to TRD participants under placebo. Preliminary findings suggest that these effects were largely observed in the absence of inflammatory (C-reactive protein) changes and were associated with both acute and sustained improvements in symptoms in the TRD group. Ketamine thus normalized fronto-striatal connectivity in TRD participants but disrupted it in HVs independently of inflammatory processes. These findings highlight the potential importance of reward circuitry in ketamine's mechanism of action, which may be particularly relevant for understanding ketamine-induced shifts in motivational symptoms.
Trial registration: ClinicalTrials.gov NCT00088699.
© 2020. The Author(s).
Conflict of interest statement
CAZ is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2
Figures

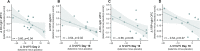
Similar articles
-
Preliminary evidence that ketamine alters anterior cingulate resting-state functional connectivity in depressed individuals.Transl Psychiatry. 2023 Dec 1;13(1):371. doi: 10.1038/s41398-023-02674-1. Transl Psychiatry. 2023. PMID: 38040678 Free PMC article. Clinical Trial.
-
Functional changes in sleep-related arousal after ketamine administration in individuals with treatment-resistant depression.Transl Psychiatry. 2024 Jun 4;14(1):238. doi: 10.1038/s41398-024-02956-2. Transl Psychiatry. 2024. PMID: 38834540 Free PMC article. Clinical Trial.
-
Ketamine Alters Electrophysiological Responses to Emotional Faces in Major Depressive Disorder.J Affect Disord. 2021 Jan 15;279:239-249. doi: 10.1016/j.jad.2020.10.007. Epub 2020 Oct 7. J Affect Disord. 2021. PMID: 33074143 Free PMC article. Clinical Trial.
-
Overlap in the neural circuitry and molecular mechanisms underlying ketamine abuse and its use as an antidepressant.Behav Brain Res. 2020 Apr 20;384:112548. doi: 10.1016/j.bbr.2020.112548. Epub 2020 Feb 13. Behav Brain Res. 2020. PMID: 32061748 Free PMC article. Review.
-
Chronic stress pathology and ketamine-induced alterations in functional connectivity in major depressive disorder: An abridged review of the clinical evidence.Adv Pharmacol. 2020;89:163-194. doi: 10.1016/bs.apha.2020.04.003. Epub 2020 May 14. Adv Pharmacol. 2020. PMID: 32616206 Review.
Cited by
-
Effects of chronic stress on cognitive function - From neurobiology to intervention.Neurobiol Stress. 2024 Sep 2;33:100670. doi: 10.1016/j.ynstr.2024.100670. eCollection 2024 Nov. Neurobiol Stress. 2024. PMID: 39295772 Free PMC article. Review.
-
Review: The use of functional magnetic resonance imaging (fMRI) in clinical trials and experimental research studies for depression.Front Neuroimaging. 2023 Jun 27;2:1110258. doi: 10.3389/fnimg.2023.1110258. eCollection 2023. Front Neuroimaging. 2023. PMID: 37554642 Free PMC article. Review.
-
Assessment of Initial Depressive State and Pain Relief With Ketamine in Patients With Chronic Refractory Pain.JAMA Netw Open. 2023 May 1;6(5):e2314406. doi: 10.1001/jamanetworkopen.2023.14406. JAMA Netw Open. 2023. PMID: 37204789 Free PMC article.
-
Functional MRI markers for treatment-resistant depression: Insights and challenges.Prog Brain Res. 2023;278:117-148. doi: 10.1016/bs.pbr.2023.04.001. Epub 2023 Jun 5. Prog Brain Res. 2023. PMID: 37414490 Free PMC article. Review.
-
The Patient's Perspective on the Effects of Intranasal Esketamine in Treatment-Resistant Depression.Brain Sci. 2023 Oct 22;13(10):1494. doi: 10.3390/brainsci13101494. Brain Sci. 2023. PMID: 37891860 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

