A dynamic CD2-rich compartment at the outer edge of the immunological synapse boosts and integrates signals
- PMID: 32929275
- PMCID: PMC7611174
- DOI: 10.1038/s41590-020-0770-x
A dynamic CD2-rich compartment at the outer edge of the immunological synapse boosts and integrates signals
Erratum in
-
Author Correction: A dynamic CD2-rich compartment at the outer edge of the immunological synapse boosts and integrates signals.Nat Immunol. 2021 Jan;22(1):99. doi: 10.1038/s41590-020-00825-w. Nat Immunol. 2021. PMID: 33122852 No abstract available.
Abstract
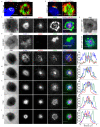
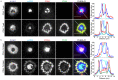
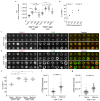
The CD2-CD58 recognition system promotes adhesion and signaling and counters exhaustion in human T cells. We found that CD2 localized to the outer edge of the mature immunological synapse, with cellular or artificial APC, in a pattern we refer to as a 'CD2 corolla'. The corolla captured engaged CD28, ICOS, CD226 and SLAM-F1 co-stimulators. The corolla amplified active phosphorylated Src-family kinases (pSFK), LAT and PLC-γ over T cell receptor (TCR) alone. CD2-CD58 interactions in the corolla boosted signaling by 77% as compared with central CD2-CD58 interactions. Engaged PD-1 invaded the CD2 corolla and buffered CD2-mediated amplification of TCR signaling. CD2 numbers and motifs in its cytoplasmic tail controlled corolla formation. CD8+ tumor-infiltrating lymphocytes displayed low expression of CD2 in the majority of people with colorectal, endometrial or ovarian cancer. CD2 downregulation may attenuate antitumor T cell responses, with implications for checkpoint immunotherapies.
Conflict of interest statement
The authors declare no competing interests
Figures




References
-
- Sanders ME, et al. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988;140:1401–1407. - PubMed
-
- Davis SJ, van der Merwe PA. The kinetic-segregation model: TCR triggering and beyond. Nature immunology. 2006;7:803–809. - PubMed
-
- Carmo AM, Mason DW, Beyers AD. Physical association of the cytoplasmic domain of CD2 with the tyrosine kinases p56lck and p59fyn. European journal of immunology. 1993;23:2196–2201. - PubMed
-
- Dustin ML, et al. A novel adapter protein orchestrates receptor patterning and cytoskeletal polarity in T cell contacts. Cell. 1998;94:667–677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

