Bacteria responsive polyoxometalates nanocluster strategy to regulate biofilm microenvironments for enhanced synergetic antibiofilm activity and wound healing
- PMID: 32929332
- PMCID: PMC7481423
- DOI: 10.7150/thno.49008
Bacteria responsive polyoxometalates nanocluster strategy to regulate biofilm microenvironments for enhanced synergetic antibiofilm activity and wound healing
Abstract
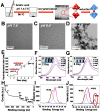
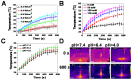
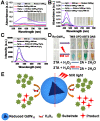
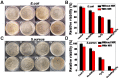
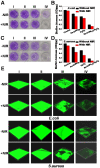
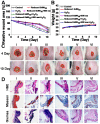
Backgroud: Nowadays, biofilms that are generated as a result of antibiotic abuse cause serious threats to global public health. Such films are the primary factor that contributes to the failure of antimicrobial treatment. This is due to the fact that the films prevent antibiotic infiltration, escape from innate immune attacks by phagocytes and consequently generate bacterial resistance. Therefore, exploiting novel antibacterial agents or strategies is extremely urgent. Methods: Herein, we report a rational construction of a novel biofilm microenvironment (BME)-responsive antibacterial platform that is based on tungsten (W)-polyoxometalate clusters (POMs) to achieve efficient bactericidal effects. Results: On one hand, the acidity and reducibility of a BME could lead to the self-assembly of POMs to produce large aggregates, which favor biofilm accumulation and enhance photothermal conversion under near-infrared (NIR) light irradiation. On the other hand, reduced POM aggregates with BME-induced photothermal-enhanced efficiency also exhibit surprisingly high peroxidase-like activity in the catalysis of bacterial endogenous hydrogen peroxide (H2O2) to produce abundant reactive oxygen species (ROS). This enhances biofilm elimination and favors antibacterial effects. Most importantly, reduced POMs exhibit the optimal peroxidase-like activity in an acidic BME. Conclusion: Therefore, in addition to providing a prospective antibacterial agent, intelligent acid/reductive dual-responsive POMs will establish a new representative paradigm for the areas of healthcare with minimal side effects.
Keywords: Biofilm; acid/reductive-responsive; peroxidase-like activity; photothermal antibacterial effect; polyoxometalate.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Li P, Poon YF, Li W, Zhu HY, Yeap SH, Cao Y. et al. A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat Mater. 2011;10:149–56. - PubMed
-
- Teng CP, Zhou T, Ye E, Liu S, Koh LD, Low M. et al. Effective targeted photothermal ablation of multidrug resistant bacteria and their biofilms with NIR-absorbing gold nanocrosses. Adv Healthc Mater. 2016;5:2122–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

