Palladium-based nanomaterials for cancer imaging and therapy
- PMID: 32929334
- PMCID: PMC7481408
- DOI: 10.7150/thno.45990
Palladium-based nanomaterials for cancer imaging and therapy
Abstract
In recent decade, palladium-based (Pd-based) nanomaterials have shown significant potential for biomedical applications because of their unique optical properties, excellent biocompatibility and high stability in physiological environment. Compared with other intensively studied noble nanomaterials, such as gold (Au) and silver (Ag) nanomaterials, research on Pd-based nanomaterials started late, but the distinctive features, such as high photothermal conversion efficiency and high photothermal stability, have made them getting great attention in the field of nanomedicine. The goal of this review is to provide a comprehensive and critical perspective on the recent progress of Pd-based nanomaterials as imaging contrast agents and therapeutic agents. The imaging section focuses on applications in photoacoustic (PA) imaging, single-photon emission computed tomography (SPECT) imaging, computed tomography (CT) imaging and magnetic resonance (MR) imaging. For treatment of cancer, single photothermal therapy (PTT) and PTT combined with other therapeutic modalities will be discussed. Finally, the safety concerns, forthcoming challenges and perspective of Pd-based nanomaterials on biomedical applications will be presented.
Keywords: Palladium-based nanomaterials; cancer imaging; combined cancer therapy; photothermal therapy; safety profile.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
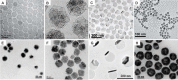
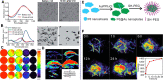





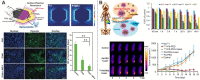

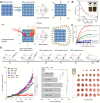


References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. - PubMed
-
- Lammers T, Aime S, Hennink WE, Storm G, Kiessling F. Theranostic nanomedicine. Acc Chem Res. 2011;44:1029–38. - PubMed
-
- Huo S, Ma H, Huang K, Liu J, Wei T, Jin S. et al. Superior penetration and retention behavior of 50 nm gold nanoparticles in tumors. Cancer Res. 2013;73:319–30. - PubMed
-
- Kim D, Shin K, Kwon SG, Hyeon T. Synthesis and biomedical applications of multifunctional nanoparticles. Adv Mater. 2018;30:1802309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

