Phorbol ester activates human mesenchymal stem cells to inhibit B cells and ameliorate lupus symptoms in MRL. Faslpr mice
- PMID: 32929342
- PMCID: PMC7481409
- DOI: 10.7150/thno.46835
Phorbol ester activates human mesenchymal stem cells to inhibit B cells and ameliorate lupus symptoms in MRL. Faslpr mice
Abstract
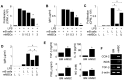
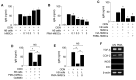
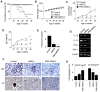
Rationale: Systemic lupus erythematosus (SLE) is a multi-organ autoimmune disease characterized by autoantibody production by hyper-activated B cells. Although mesenchymal stem cells (MSCs) ameliorate lupus symptoms by inhibiting T cells, whether they inhibit B cells has been controversial. Here we address this issue and reveal how to prime MSCs to inhibit B cells and improve the efficacy of MSCs in SLE. Methods: We examined the effect of MSCs on purified B cells in vitro and the therapeutic efficacy of MSCs in lupus-prone MRL.Faslpr mice. We screened chemicals for their ability to activate MSCs to inhibit B cells. Results: Mouse bone marrow-derived MSCs inhibited mouse B cells in a CXCL12-dependent manner, whereas human bone marrow-derived MSCs (hMSCs) did not inhibit human B (hB) cells. We used a chemical approach to overcome this hurdle and found that phorbol myristate acetate (PMA), phorbol 12,13-dibutyrate, and ingenol-3-angelate rendered hMSCs capable of inhibiting IgM production by hB cells. As to the mechanism, PMA-primed hMSCs attracted hB cells in a CXCL10-dependent manner and induced hB cell apoptosis in a PD-L1-dependent manner. Finally, we showed that PMA-primed hMSCs were better than naïve hMSCs at ameliorating SLE progression in MRL.Faslpr mice. Conclusion: Taken together, our data demonstrate that phorbol esters might be good tool compounds to activate MSCs to inhibit B cells and suggest that our chemical approach might allow for improvements in the therapeutic efficacy of hMSCs in SLE.
Keywords: B cell; CXCL10; PD-L1; mesenchymal stem cell; phorbol ester; systemic lupus erythematosus.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Morel L. Immunometabolism in systemic lupus erythematosus. Nat Rev Rheumatol. 2017;13:280–90. - PubMed
-
- Lee JH, Lee HK, Kim HS, Kim JS, Ji AY, Lee JS. et al. CXCR3-deficient mesenchymal stem cells fail to infiltrate into the nephritic kidney and do not ameliorate lupus symptoms in MRL. Fas(lpr) mice. Lupus. 2018;27:1854–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

