Dihydroartemisinin ameliorates psoriatic skin inflammation and its relapse by diminishing CD8+ T-cell memory in wild-type and humanized mice
- PMID: 32929360
- PMCID: PMC7482806
- DOI: 10.7150/thno.45211
Dihydroartemisinin ameliorates psoriatic skin inflammation and its relapse by diminishing CD8+ T-cell memory in wild-type and humanized mice
Abstract
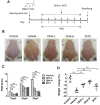
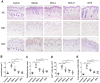
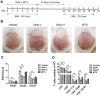
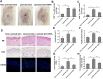
Conventional immunosuppressants cause side effects and do not prevent the recurrence of autoimmune diseases. Moreover, they may not inhibit autoimmunity mediated by pathogenic memory T-cells. Dihydroartemisinin (DHA) has been shown to regulate autoimmunity. However, it remains unknown whether DHA impacts psoriasis and its recurrence. The objective of this study was to determine therapeutic effects of DHA on psoriasis and its relapse as well as its underlying mechanisms. Methods: We established animal models of imiquimod (IMQ)-induced psoriasis-like wild-type mice and humanized NSG mice receiving lesional human skin from patients with psoriasis. Many immunoassays, including immunohistochemistry, flow cytometry, quantitative RT-PCR and Western blotting, were performed. Results: We found that DHA not only ameliorated acute skin lesion of psoriatic mice, but also alleviated its recurrence by diminishing CD8+ central memory T (TCM) and CD8+ resident memory T (TRM) cells. It attenuated epidermal pathology and T-cell infiltration in the skin of IMQ-induced psoriatic mice while suppressing expression of IL-15, IL-17 and other proinflammatory cytokines in the skin. Surprisingly, DHA reduced the frequency and number of CD8+, but not CD4+, subset of CD44highCD62Lhigh TCM in psoriatic mice, whereas methotrexate (MTX) lowered CD4+, but not CD8+, TCM frequency and number. Indeed, DHA, but not MTX, downregulated eomesodermin (EOMES) and BCL-6 expression in CD8+ T-cells. Furthermore, DHA, but not MTX, reduced the presence of CD8+CLA+, CD8+CD69+ or CD8+CD103+ TRM cells in mouse skin. Interestingly, treatment with DHA, but not MTX, during the first onset of psoriasis largely prevented psoriasis relapse induced by low doses of IMQ two weeks later. Administration of recombinant IL-15 or CD8+, but not CD4+, TCM cells resulted in complete recurrence of psoriasis in mice previously treated with DHA. Finally, we demonstrated that DHA alleviated psoriatic human skin lesions in humanized NSG mice grafted with lesional skin from psoriatic patients while reducing human CD8+ TCM and CD103+ TRM cells in humanized mice. Conclusion: We have provided the first evidence that DHA is advantageous over MTX in preventing psoriasis relapse by reducing memory CD8+ T-cells.
Keywords: Dihydroartemisinin; Immunosuppression; Memory T cells; Psoriasis; Relapse.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Di TT, Ruan ZT, Zhao JX, Wang Y, Liu X, Wang Y. et al. Astilbin inhibits Th17 cell differentiation and ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice via Jak3/Stat3 signaling pathway. Int Immunopharmacol. 2016;32:32–38. - PubMed
-
- Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu Rev Pathol. 2012;7:385–422. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

