A Systematic Review of Treatment and Outcomes of Pregnant Women With COVID-19-A Call for Clinical Trials
- PMID: 32929403
- PMCID: PMC7454907
- DOI: 10.1093/ofid/ofaa350
A Systematic Review of Treatment and Outcomes of Pregnant Women With COVID-19-A Call for Clinical Trials
Abstract
Background: Data pertaining to COVID-19 in pregnancy are limited; to better inform clinicians, we collated data from COVID-19 cases during pregnancy and summarized clinical trials enrolling this population.
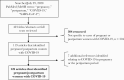
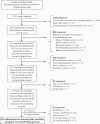
Methods: We performed a systematic literature review of PubMed/MEDLINE to identify cases of COVID-19 in pregnancy or the postpartum period and associated outcomes. We then evaluated the proportion of COVID-19 clinical trials (from ClinicalTrials.gov) excluding pregnant or breastfeeding persons (both through June 29, 2020).
Results: We identified 11 308 published cases of COVID-19 during pregnancy. Of those reporting disease severity, 21% (416/1999) were severe/critical. Maternal and neonatal survival were reassuring (98% [10 437/10 597] and 99% [1155/1163], respectively). Neonatal disease was rare, with only 41 possible cases of infection reported in the literature. Of 2351 ongoing COVID-19 therapeutic clinical trials, 1282 were enrolling persons of reproductive age and 65% (829/1282) excluded pregnant persons. Pregnancy was an exclusion criterion for 69% (75/109) of chloroquine/hydroxychloroquine, 80% (28/35) of lopinavir/ritonavir, and 48% (44/91) of convalescent plasma studies. We identified 48 actively recruiting or completed drug trials reporting inclusion of this population.
Conclusions: There are limited published reports of COVID-19 in pregnancy despite more than 14 million cases worldwide. To date, clinical outcomes appear reassuring, but data related to important long-term outcomes are missing or not yet reported. The large number of clinical trials excluding pregnant persons, despite interventions with safety data in pregnancy, is concerning. In addition to observational cohort studies, pregnancy-specific adaptive clinical trials could be designed to identify safe and effective treatments.
Keywords: COVID-19; SARS-CoV-2; breastfeeding; coronavirus; pregnancy.
© The Author(s) 2020. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures
References
-
- World Health Organization. Women of reproductive age (15–49 years) population (thousands). Available at: https://www.who.int/data/maternal-newborn-child-adolescent/indicator-exp...). Accessed 20 April 2020.
-
- World Health Organization. Women in the health workforce. Available at: https://www.who.int/hrh/events/2018/women-in-health-workforce/en/. Accessed 20 April 2020.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous