Bone marrow regeneration requires mitochondrial transfer from donor Cx43-expressing hematopoietic progenitors to stroma
- PMID: 32929449
- PMCID: PMC7735163
- DOI: 10.1182/blood.2020005399
Bone marrow regeneration requires mitochondrial transfer from donor Cx43-expressing hematopoietic progenitors to stroma
Abstract
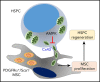
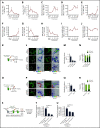
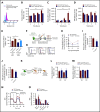
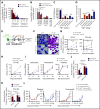
The fate of hematopoietic stem and progenitor cells (HSPC) is tightly regulated by their bone marrow (BM) microenvironment (ME). BM transplantation (BMT) frequently requires irradiation preconditioning to ablate endogenous hematopoietic cells. Whether the stromal ME is damaged and how it recovers after irradiation is unknown. We report that BM mesenchymal stromal cells (MSC) undergo massive damage to their mitochondrial function after irradiation. Donor healthy HSPC transfer functional mitochondria to the stromal ME, thus improving mitochondria activity in recipient MSC. Mitochondrial transfer to MSC is cell-contact dependent and mediated by HSPC connexin-43 (Cx43). Hematopoietic Cx43-deficient chimeric mice show reduced mitochondria transfer, which was rescued upon re-expression of Cx43 in HSPC or culture with isolated mitochondria from Cx43 deficient HSPCs. Increased intracellular adenosine triphosphate levels activate the purinergic receptor P2RX7 and lead to reduced activity of adenosine 5'-monophosphate-activated protein kinase (AMPK) in HSPC, dramatically increasing mitochondria transfer to BM MSC. Host stromal ME recovery and donor HSPC engraftment were augmented after mitochondria transfer. Deficiency of Cx43 delayed mesenchymal and osteogenic regeneration while in vivo AMPK inhibition increased stromal recovery. As a consequence, the hematopoietic compartment reconstitution was improved because of the recovery of the supportive stromal ME. Our findings demonstrate that healthy donor HSPC not only reconstitute the hematopoietic system after transplantation, but also support and induce the metabolic recovery of their irradiated, damaged ME via mitochondria transfer. Understanding the mechanisms regulating stromal recovery after myeloablative stress are of high clinical interest to optimize BMT procedures and underscore the importance of accessory, non-HSC to accelerate hematopoietic engraftment.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


Comment in
-
HSCs revive their niche after transplantation.Blood. 2020 Dec 3;136(23):2597-2598. doi: 10.1182/blood.2020008923. Blood. 2020. PMID: 33270855 No abstract available.
References
-
- Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. 2015;16(3):239-253. - PubMed
-
- Abbuehl JP, Tatarova Z, Held W, Huelsken J. Long-term engraftment of primary bone marrow stromal cells repairs niche damage and improves hematopoietic stem cell transplantation. Cell Stem Cell. 2017;21(2):241-255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

